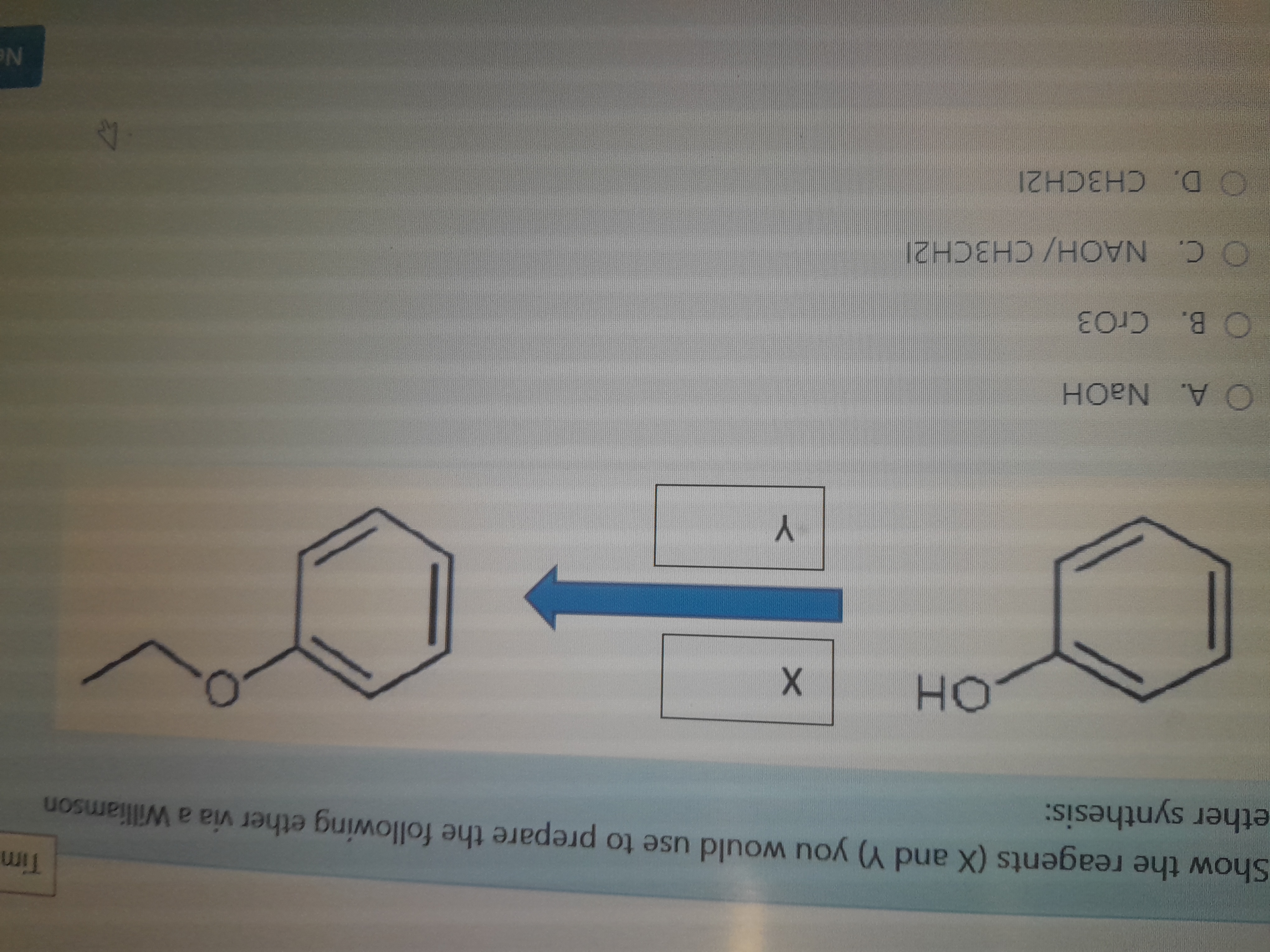
Show the reagents (X and Y) you would use to prepare the following ether via a Williamson ether synthesis.
Understand the Problem
The question is asking to identify the reagents (X and Y) needed for the synthesis of a specific ether using the Williamson ether synthesis method. It provides a molecular structure and a set of possible reagent choices to select from.
Answer
NaOH and CH3CH2I
The reagents are NaOH and CH3CH2I.
Answer for screen readers
The reagents are NaOH and CH3CH2I.
More Information
The Williamson ether synthesis involves the reaction of an alkoxide ion with an alkyl halide. Here, the phenoxide ion reacts with ethyl iodide to form phenyl ethyl ether.
Tips
A common mistake is using a primary alcohol instead of the correct alkyl halide. The halide should be the less hindered, good leaving group.
Sources
- The Williamson Ether Synthesis - Master Organic Chemistry - masterorganicchemistry.com
- Williamson Ether Synthesis - Mechanism, Uses, Limitations - BYJU'S - byjus.com
AI-generated content may contain errors. Please verify critical information