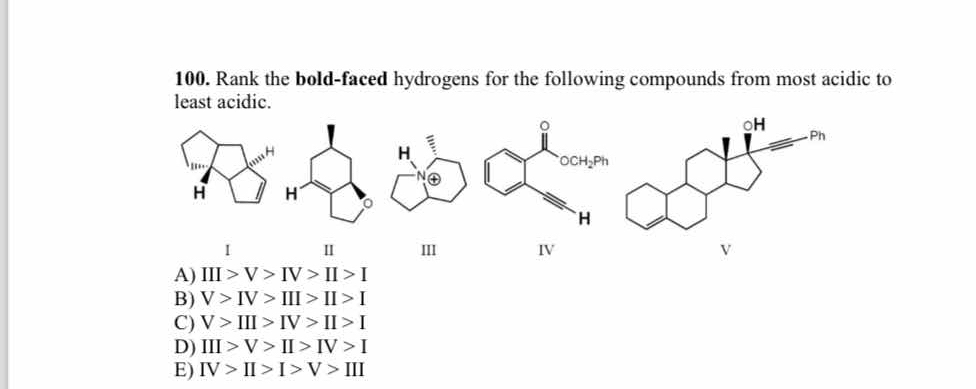
Rank the bold-faced hydrogens for the following compounds from most acidic to least acidic.
Understand the Problem
The question is asking to rank the bold-faced hydrogens of the given compounds based on their acidity, from most acidic to least acidic. This will involve analyzing the chemical structures and understanding the factors affecting acidity such as resonance, electronegativity, and molecular stability.
Answer
V > IV > III > II > I
The ranking from most acidic to least acidic is V > IV > III > II > I.
Answer for screen readers
The ranking from most acidic to least acidic is V > IV > III > II > I.
More Information
The acidity of the hydrogen atoms depends on their ability to donate protons, influenced by the electron-withdrawing nature of nearby atoms and the stability of the resulting conjugate base.
Tips
Always consider the inductive effect and resonance stabilization when assessing acidity.
Sources
- Rank the bold-faced hydrogens for the following compounds - brainly.com
- Rank the bold-faced hydrogens for the following compounds - Course Hero - coursehero.com
AI-generated content may contain errors. Please verify critical information