Propose the mechanism.
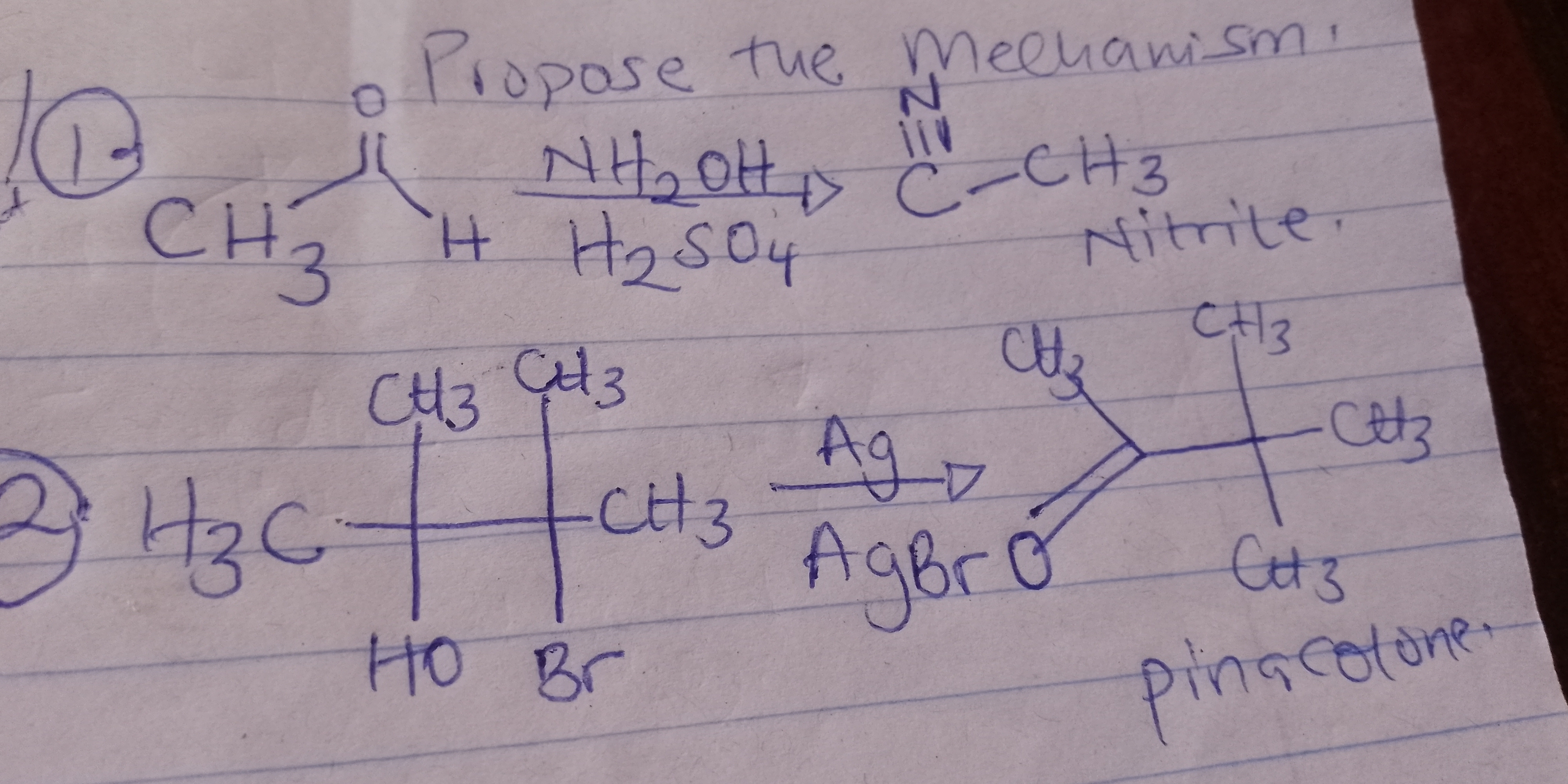
Understand the Problem
The question is asking to propose a chemical mechanism for the reactions shown in the image, specifically involving the conversion of certain reactants into products including a nitrile and pinacolone. This requires knowledge of organic chemistry mechanisms and the specific reagents involved.
Answer
Oxime to nitrile via acid, bromohydrin rearrangement with Ag to pinacolone.
For CH3 with NH2OH and H2SO4, form oxime then nitrile. For CH3 with HOBr, silver facilitates AgBr formation, leading to pinacolone.
Answer for screen readers
For CH3 with NH2OH and H2SO4, form oxime then nitrile. For CH3 with HOBr, silver facilitates AgBr formation, leading to pinacolone.
More Information
Reaction mechanisms involve stepwise transformations. Reaction 1 includes oxime formation and rearrangement to a nitrile. Reaction 2 involves a silver-catalyzed rearrangement to form a ketone.
Tips
Ensure the protonation and leaving groups are correctly assigned in each step. Misidentifying intermediates can lead to incorrect mechanisms.
Sources
- 14.6: Reaction Mechanisms - Chemistry LibreTexts - chem.libretexts.org
AI-generated content may contain errors. Please verify critical information