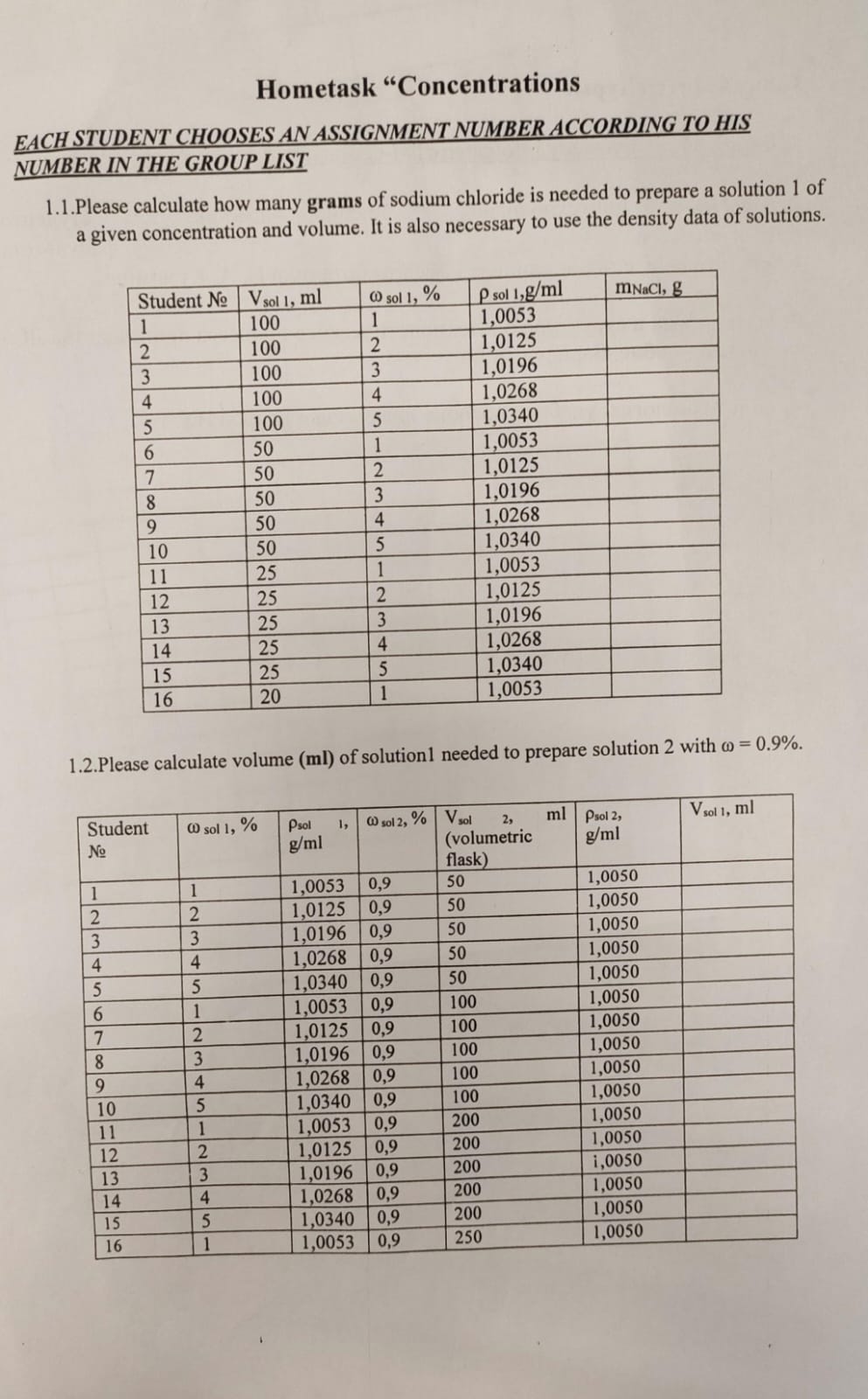
Please calculate how many grams of sodium chloride is needed to prepare a solution of a given concentration and volume. Also, calculate the volume of solution needed to prepare sol... Please calculate how many grams of sodium chloride is needed to prepare a solution of a given concentration and volume. Also, calculate the volume of solution needed to prepare solution 2 with a weight percentage of 0.9%.
Understand the Problem
The question is asking for the calculations of grams of sodium chloride needed for specific concentrations and volumes for student assignments. It also requires the calculation of the volume of solution needed to prepare a second solution with a given concentration.
Answer
Mass of sodium chloride for Student 1: $1.0053 \, \text{g}$; Volume of solution 1 needed for solution 2: $44.77 \, \text{ml}$.
Answer for screen readers
For Student 1, the mass of sodium chloride needed is approximately (1.0053 , \text{g}) and (V_{\text{sol 1}} \approx 44.77 , \text{ml}).
Steps to Solve
- Identifying the Variables Identify the relevant variables from the table for the calculations. For example, for Student 1 from the first part, we have:
- (V_{\text{sol 1}} = 100 , \text{ml})
- (\omega_{\text{sol 1}} = 1%)
- (\rho_{\text{sol 1}} = 1.0053 , \text{g/ml})
-
Calculating Mass of Sodium Chloride To find the mass of sodium chloride needed for preparation, use the formula: $$ \text{mass} = \left(\frac{\omega_{\text{sol 1}}}{100}\right) \times V_{\text{sol 1}} \times \rho_{\text{sol 1}} $$ Substituting the values for Student 1: $$ \text{mass} = \left(\frac{1}{100}\right) \times 100 , \text{ml} \times 1.0053 , \text{g/ml} = 1.0053 , \text{g} $$
-
Repeating for Other Students Repeat the calculation for each student by using their respective values from the table under (V_{\text{sol 1}}, \omega_{\text{sol 1}},) and (\rho_{\text{sol 1}}).
-
Volume of Solution Required for Second Solution For the second part (1.2), to find the volume of solution 1 needed to prepare solution 2 with (\omega = 0.9%): Use the rearranged formula: $$ V_{\text{sol 1}} = \frac{\omega_{\text{sol 2}} \times V_{\text{sol 2}}}{\omega_{\text{sol 1}} \times \rho_{\text{sol 1}}} $$ Using the values from the table: For Student 1, with (V_{\text{sol 2}} = 50 , \text{ml}): $$ V_{\text{sol 1}} = \frac{0.9 \times 50}{1 \times 1.0053} \approx 44.77 , \text{ml} $$
-
Final Review Be sure to review calculations for each student to ensure accuracy.
For Student 1, the mass of sodium chloride needed is approximately (1.0053 , \text{g}) and (V_{\text{sol 1}} \approx 44.77 , \text{ml}).
More Information
The mass of sodium chloride is calculated based on the percentage concentration and the volume of the solution. The volume for the second solution is calculated by applying a proportion based on the desired concentration.
Tips
- Confusing mass and volume: Make sure to differentiate between grams and milliliters.
- Forgetting to convert percentages: Always convert the percentage to a decimal when using it in calculations.
AI-generated content may contain errors. Please verify critical information