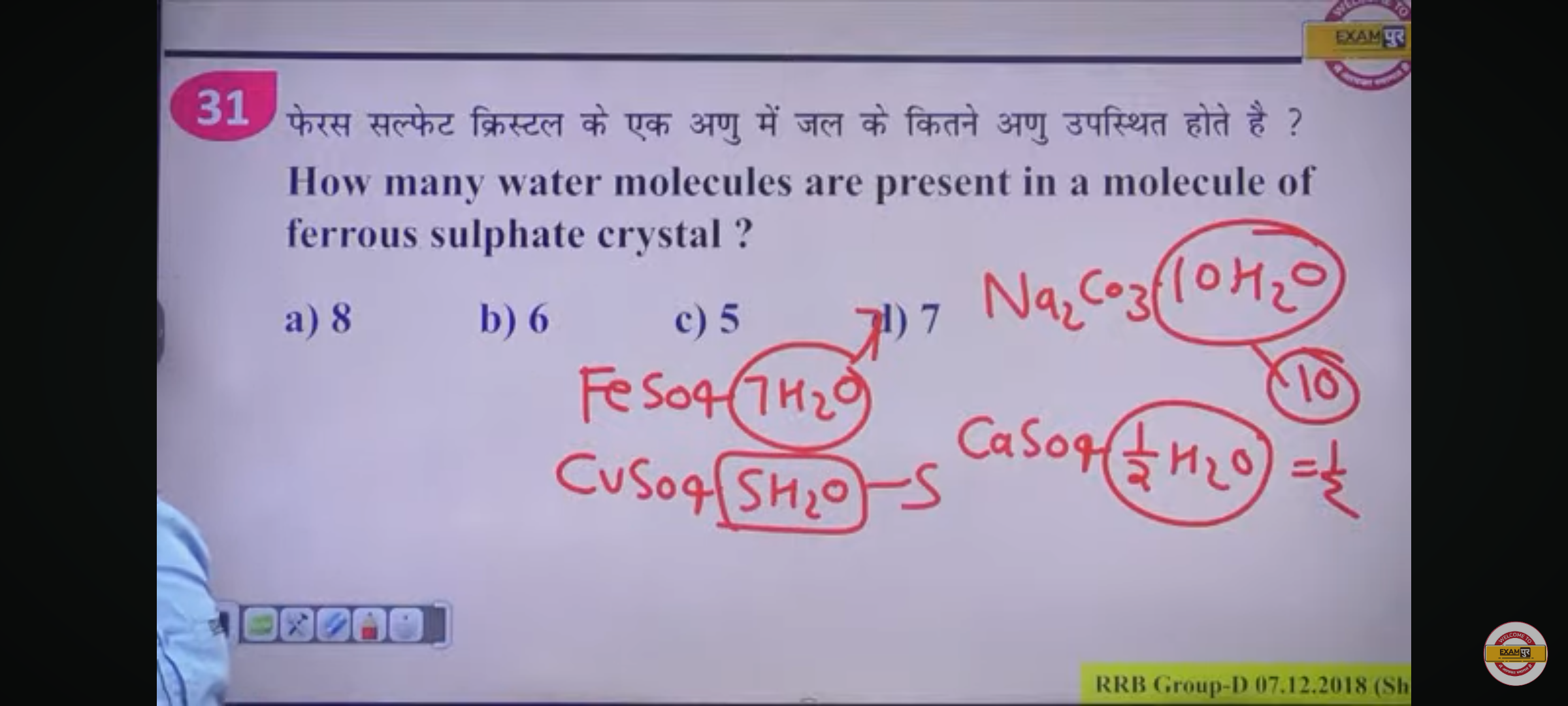
How many water molecules are present in a molecule of ferrous sulphate crystal?
Understand the Problem
The question is asking for the number of water molecules associated with a molecule of ferrous sulfate crystal, which is usually represented as FeSO4·nH2O. This is a chemistry-related question that involves understanding the molecular formula of ferrous sulfate.
Answer
The number of water molecules in ferrous sulfate crystal is \( 7 \).
Answer for screen readers
The number of water molecules present in a molecule of ferrous sulfate crystal is ( n = 7 ).
Steps to Solve
- Identify the Water Molecules in the Formula
The molecular formula for ferrous sulfate with water is given as $FeSO_4 \cdot nH_2O$.
- Find the Value of n
From the problem, it is given that for ferrous sulfate, the value of $n$ equals 7, meaning there are 7 water molecules associated with each molecule.
- Write the Conclusion
Here, $FeSO_4 \cdot 7H_2O$ indicates that there are a total of 7 water molecules in the crystal structure of ferrous sulfate.
The number of water molecules present in a molecule of ferrous sulfate crystal is ( n = 7 ).
More Information
Ferrous sulfate, or iron(II) sulfate, commonly forms crystals that are typically hydrated with 7 water molecules, giving it the appearance of blue-green crystals. This property is commonly recognized in many educational contexts.
Tips
- A common mistake might be to confuse ferrous sulfate with other sulfate compounds which may have different numbers of associated water molecules, like copper(II) sulfate which has 5.
- Ensure to correctly identify the specific hydrated form of each compound.
AI-generated content may contain errors. Please verify critical information