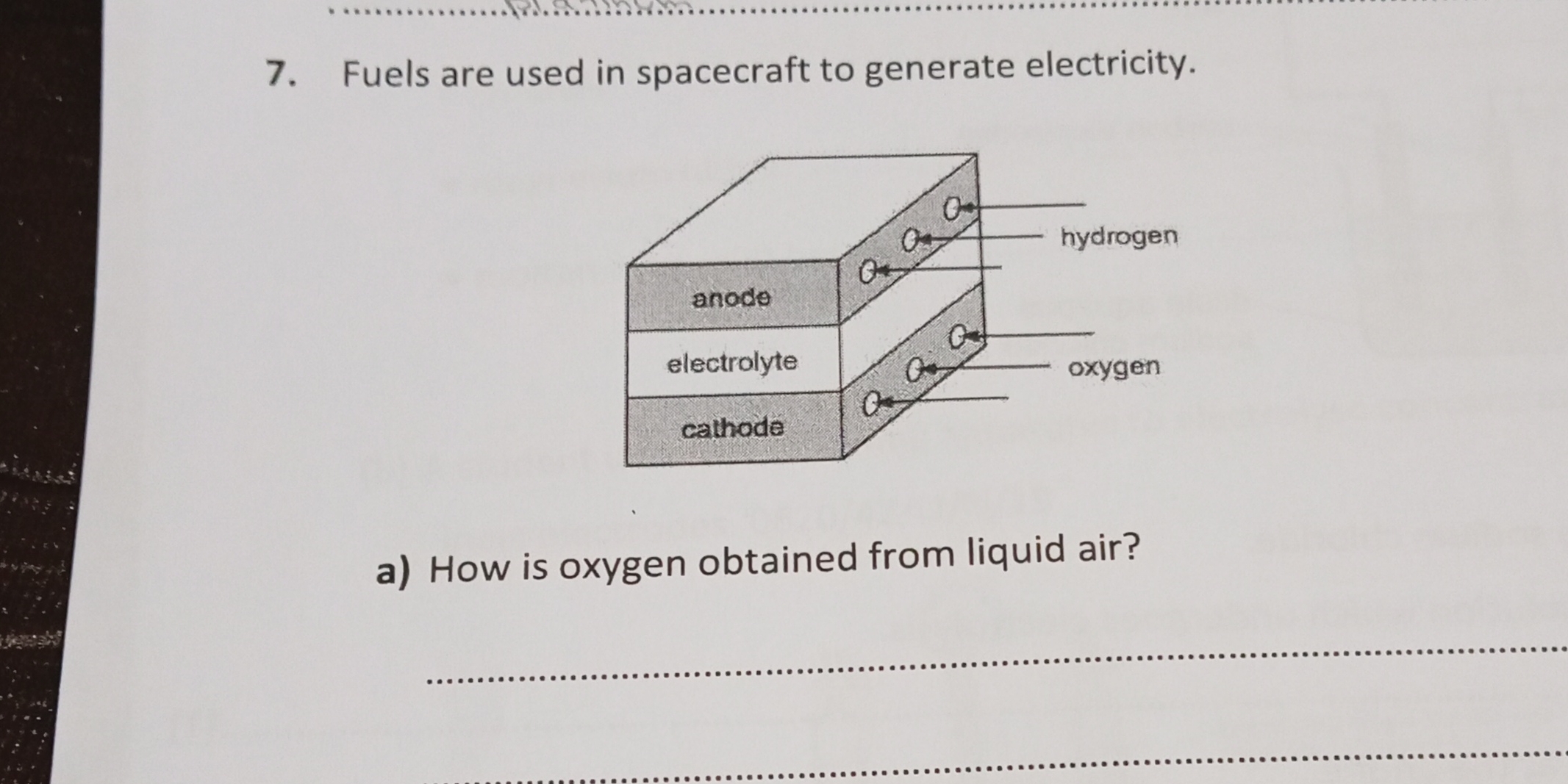
How is oxygen obtained from liquid air?
Understand the Problem
The question is asking how oxygen can be extracted from liquid air, which involves a process or method related to the properties of air and its components at low temperatures.
Answer
Liquid air is distilled fractionally to separate oxygen.
Oxygen is obtained from liquid air through fractional distillation. Air is cooled and compressed to become liquid, and then gradually warmed in a fractionating column. Gases separate based on boiling points: nitrogen vaporizes first, leaving liquid oxygen.
Answer for screen readers
Oxygen is obtained from liquid air through fractional distillation. Air is cooled and compressed to become liquid, and then gradually warmed in a fractionating column. Gases separate based on boiling points: nitrogen vaporizes first, leaving liquid oxygen.
More Information
Fractional distillation leverages differences in boiling points to separate gases. Oxygen's higher boiling point than nitrogen makes it remain as a liquid after nitrogen is distilled off.
Tips
A common mistake is overlooking the necessity of cooling air enough to liquefy it before distillation.
Sources
AI-generated content may contain errors. Please verify critical information