H2 reacts with trans-(Ph3P)2Ir(CO)Cl to primarily produce __.
Understand the Problem
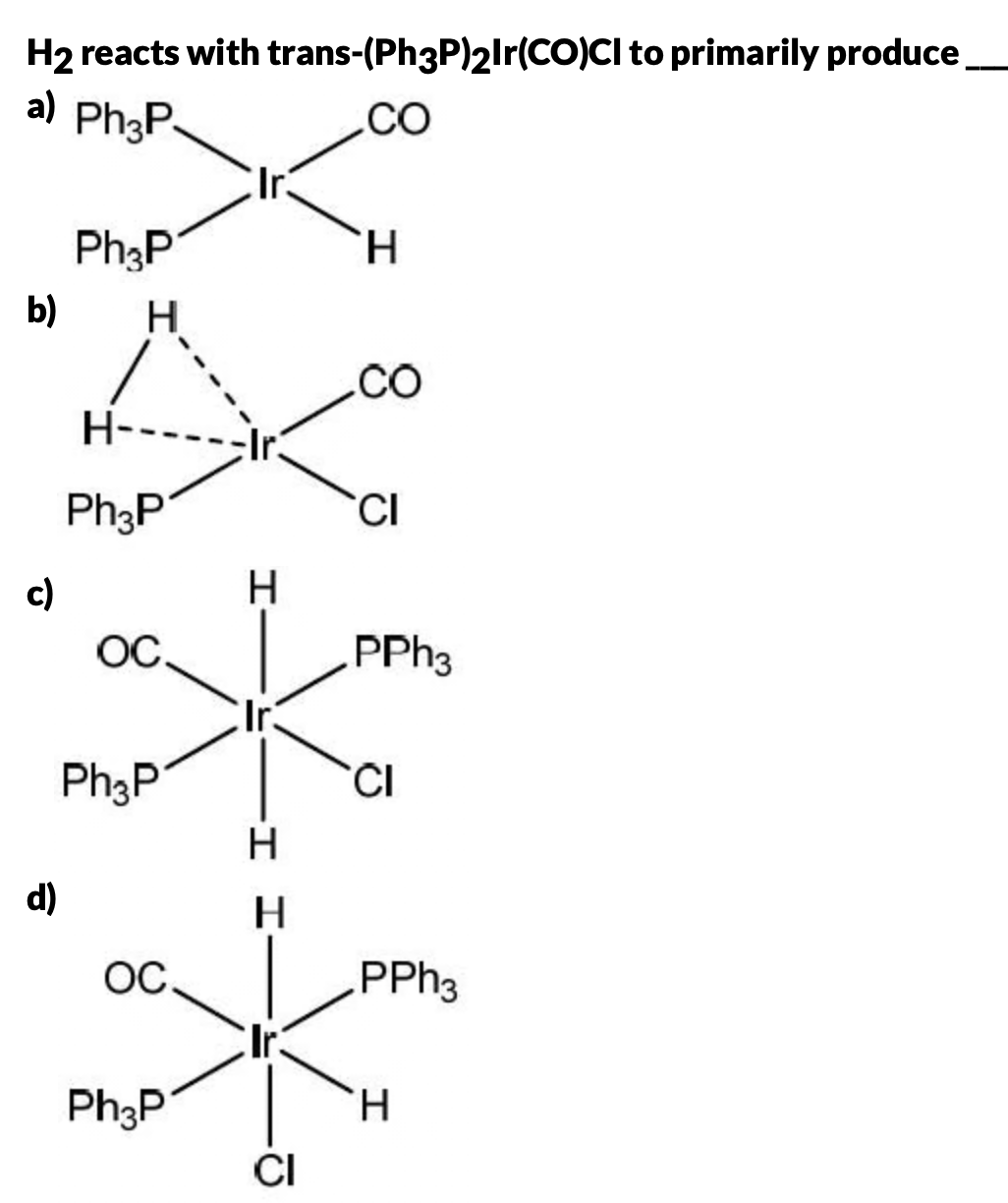
The question is asking what product forms when H2 reacts with the given iridium complex trans-(Ph3P)2Ir(CO)Cl. It involves understanding coordination chemistry and likely requires knowledge of the expected outcome of hydrogenation reactions involving transition metal complexes.
Answer
Option 'd'.
The final answer is option 'd'.
Answer for screen readers
The final answer is option 'd'.
More Information
The reaction of H2 with trans-(Ph3P)2Ir(CO)Cl, a version of Vaska's complex, typically results in the oxidative addition of H2, forming a dihydrido complex, shown in option 'd'.
Tips
Be careful to consider the correct orientation and bonds formed during oxidative addition reactions.
Sources
AI-generated content may contain errors. Please verify critical information