For the reaction C2H6(g) + H2(g) ⇌ 2 CH4(g), the standard change in Gibbs free energy is ΔG° = -32.8 kJ/mol. What is ΔG for this reaction at 298 K when the partial pressures are P_... For the reaction C2H6(g) + H2(g) ⇌ 2 CH4(g), the standard change in Gibbs free energy is ΔG° = -32.8 kJ/mol. What is ΔG for this reaction at 298 K when the partial pressures are P_C2H6 = 0.300 atm, P_H2 = 0.400 atm, and P_CH4 = 0.900 atm?
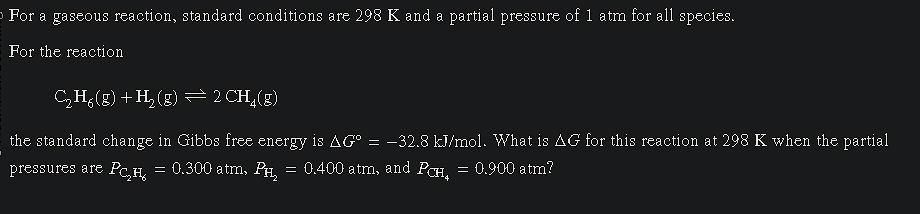
Understand the Problem
The question is asking for the Gibbs free energy change (ΔG) for the chemical reaction at specified partial pressures. To solve it, we will use the standard Gibbs free energy change and apply the relationship between ΔG and the reaction quotient Q, taking into account the given partial pressures.
Answer
The Gibbs free energy change for the reaction is ΔG ≈ $-28.1 \, \text{kJ/mol}$.
Answer for screen readers
The Gibbs free energy change, ΔG, for the reaction is approximately $-28.1 , \text{kJ/mol}$.
Steps to Solve
- Determine the Reaction Quotient (Q)
The reaction quotient $Q$ is calculated using the formula:
$$ Q = \frac{(P_{CH_4})^2}{(P_{C_2H_6})(P_{H_2})} $$
Substituting the given partial pressures:
$$ Q = \frac{(0.900)^2}{(0.300)(0.400)} $$
- Calculate Q Value
Now calculate the values:
$$ Q = \frac{0.81}{0.12} = 6.75 $$
- Calculate ΔG Using the Gibbs Free Energy Equation
The Gibbs free energy change can be calculated with the equation:
$$ \Delta G = \Delta G^\circ + RT \ln(Q) $$
Where:
- $\Delta G^\circ = -32.8 , \text{kJ/mol} = -32800 , \text{J/mol}$ (conversion to J)
- $R = 8.314 , \text{J/(mol·K)}$
- $T = 298 , \text{K}$
Substituting the values:
$$ \Delta G = -32800 + (8.314 \times 298) \ln(6.75) $$
- Calculate the Logarithm and Complete the Calculation
First, calculate $\ln(6.75)$:
$$ \ln(6.75) \approx 1.911 $$
Now plug this into the equation:
$$ \Delta G = -32800 + (8.314 \times 298 \times 1.911) $$
Calculate $8.314 \times 298 \approx 2477.572$:
Now compute:
$$ \Delta G \approx -32800 + 2477.572 \times 1.911 \approx -32800 + 4735.11 \approx -28064.89 , \text{J/mol} $$
Lastly, converting back to kJ:
$$ \Delta G \approx -28.1 , \text{kJ/mol} $$
The Gibbs free energy change, ΔG, for the reaction is approximately $-28.1 , \text{kJ/mol}$.
More Information
The ΔG value indicates that the reaction is still energetically favorable under the given conditions, though it is less favorable than at standard conditions.
Tips
- Forgetting to convert ΔG° from kJ to J when calculating with R.
- Miscalculating the logarithm value or the final ΔG calculation.
- Not using correct units while performing calculations, especially when dealing with pressure or energy.
AI-generated content may contain errors. Please verify critical information