Fill in the table with the condensed formulas and bond-line formulas for the given alkyl groups.
Understand the Problem
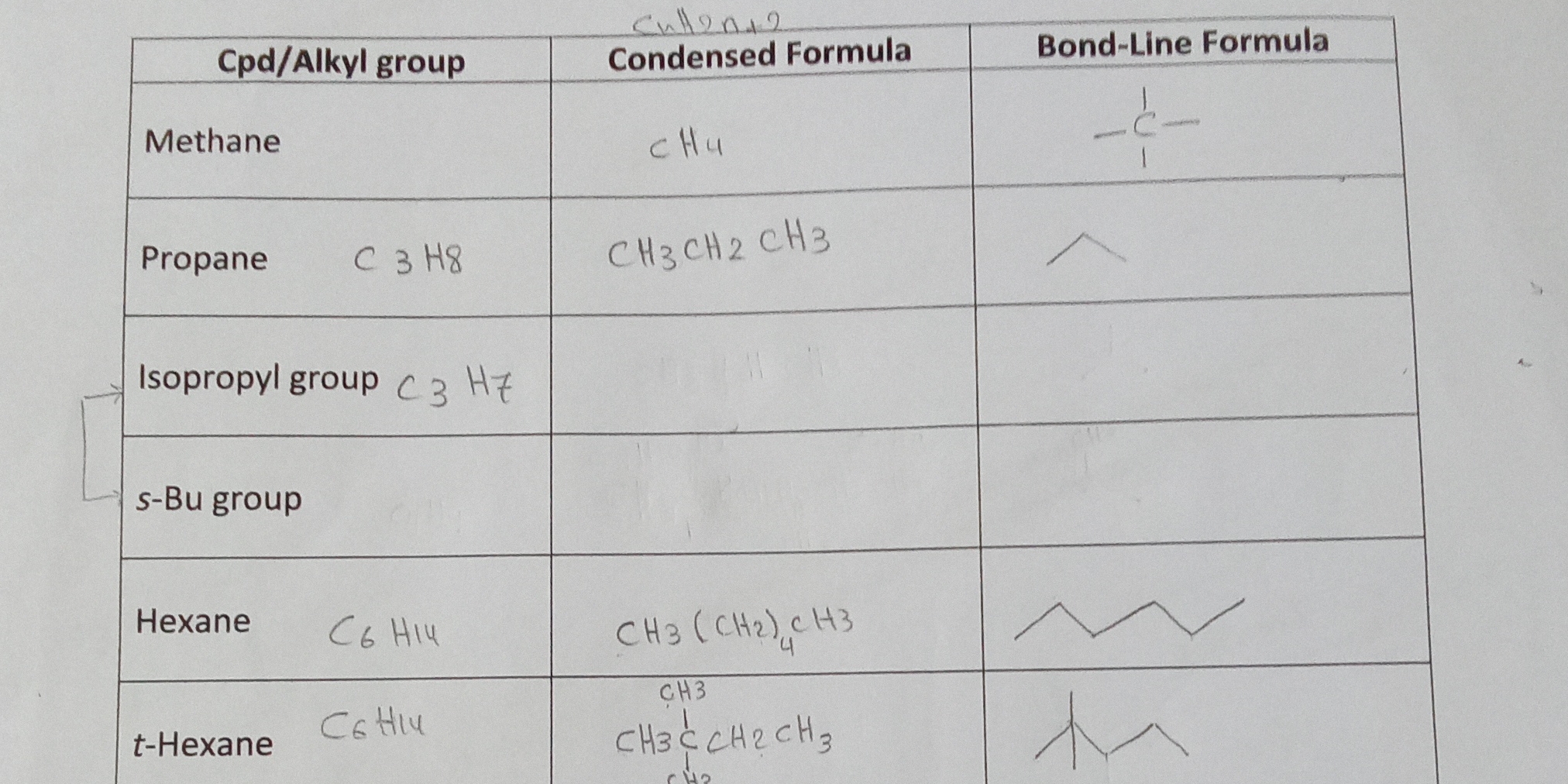
The question is presenting a table that includes various organic compounds (alkyl groups) along with their condensed and bond-line formulas. It seems to be focused on gathering or completing information related to these compounds.
Answer
Methane: a star-like symbol \n Isopropyl: 'Y' shape \n s-Bu: a 'Z' shape with branch \n t-Hexane: a branched chain
The missing bond-line formulas are: Methane: (four lines representing four Hs connected to C), isopropyl group: a branched line (like a 'Y'), s-Bu group: a zig-zag line with a branch (one 'peak' with one branch off the side), t-Hexane: a chain with branches.
Answer for screen readers
The missing bond-line formulas are: Methane: (four lines representing four Hs connected to C), isopropyl group: a branched line (like a 'Y'), s-Bu group: a zig-zag line with a branch (one 'peak' with one branch off the side), t-Hexane: a chain with branches.
More Information
Bond-line formulas are simplified representations where carbon atoms are shown at the vertices and hydrogen atoms are omitted for clarity.
Tips
A common mistake is forgetting to include branches on the correct carbon atoms in the bond-line formulas.
Sources
- 3.3: Alkyl Groups - Chemistry LibreTexts - chem.libretexts.org
- Condensed Structural Formulas: Deciphering What the Brackets Mean - masterorganicchemistry.com
AI-generated content may contain errors. Please verify critical information