Draw structures for the following: a. isopropyl propyl ether b. butyl ethyl ether c. sec-butyl methyl ether d. diisopropyl ether
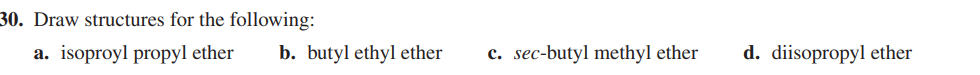
Understand the Problem
The question is asking for the structural representations of four different ether compounds: isopropyl propyl ether, butyl ethyl ether, sec-butyl methyl ether, and diisopropyl ether. This requires knowledge of organic chemistry to draw the correct molecular structures.
Answer
a. CH₃-CH(CH₃)-O-CH₂-CH₂-CH₃ b. CH₃-CH₂-CH₂-CH₂-O-CH₂-CH₃ c. CH₃-CH(CH₃)-CH₂-O-CH₃ d. (CH₃)₂CH-O-CH(CH₃)₂
The final answer is:
a. Isopropyl propyl ether: CH₃-CH(CH₃)-O-CH₂-CH₂-CH₃
b. Butyl ethyl ether: CH₃-CH₂-CH₂-CH₂-O-CH₂-CH₃
c. Sec-butyl methyl ether: CH₃-CH(CH₃)-CH₂-O-CH₃
d. Diisopropyl ether: (CH₃)₂CH-O-CH(CH₃)₂
Answer for screen readers
The final answer is:
a. Isopropyl propyl ether: CH₃-CH(CH₃)-O-CH₂-CH₂-CH₃
b. Butyl ethyl ether: CH₃-CH₂-CH₂-CH₂-O-CH₂-CH₃
c. Sec-butyl methyl ether: CH₃-CH(CH₃)-CH₂-O-CH₃
d. Diisopropyl ether: (CH₃)₂CH-O-CH(CH₃)₂
More Information
Ethers are compounds with an oxygen atom connected to two alkyl or aryl groups. Their nomenclature generally involves listing the two groups on either side of the oxygen atom followed by 'ether'.
Tips
A common mistake is confusing the structure of branched groups like isopropyl and sec-butyl.
AI-generated content may contain errors. Please verify critical information