Describe why hydrogen bonds form between water molecules. Explain why the arrangement of water molecules is different in ice and water.
Understand the Problem
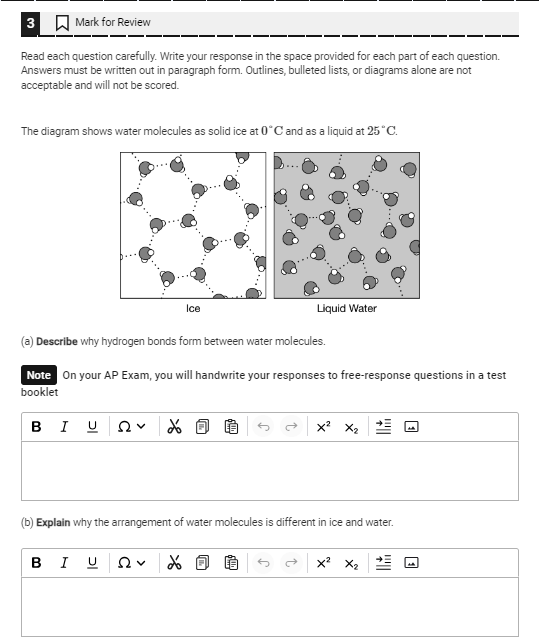
The question is asking for a description of why hydrogen bonds form between water molecules and an explanation of how the arrangement of water molecules differs between ice and liquid water. This requires an understanding of molecular structure and properties of water.
Answer
Hydrogen bonds form due to water's polarity. In ice, stable bonds form a hexagonal lattice, while in liquid water, bonds constantly rearrange.
Hydrogen bonds form between water molecules due to the polarity of water. The oxygen atom is highly electronegative, so it becomes partially negative, while the hydrogen atoms are partially positive. This causes hydrogen bonds to form between the positive hydrogen of one molecule and the negative oxygen of another. In ice, water molecules form a stable, open, hexagonal structure because the molecules slow down and the hydrogen bonds become more stable. In liquid water, the hydrogen bonds constantly break and reform, allowing the molecules to move closer together than in ice.
Answer for screen readers
Hydrogen bonds form between water molecules due to the polarity of water. The oxygen atom is highly electronegative, so it becomes partially negative, while the hydrogen atoms are partially positive. This causes hydrogen bonds to form between the positive hydrogen of one molecule and the negative oxygen of another. In ice, water molecules form a stable, open, hexagonal structure because the molecules slow down and the hydrogen bonds become more stable. In liquid water, the hydrogen bonds constantly break and reform, allowing the molecules to move closer together than in ice.
More Information
The unique properties of water, such as its high specific heat, surface tension, and expansion upon freezing, are largely due to hydrogen bonding.
Tips
Be careful not to confuse the breaking and reforming of hydrogen bonds in water with chemical bond changes. Remember that it's the arrangement and density of molecules that differentiate ice from liquid water.
Sources
- Hydrogen bonds in water (article) | Khan Academy - khanacademy.org
- 7.3: Hydrogen-Bonding and Water - Chemistry LibreTexts - chem.libretexts.org
AI-generated content may contain errors. Please verify critical information