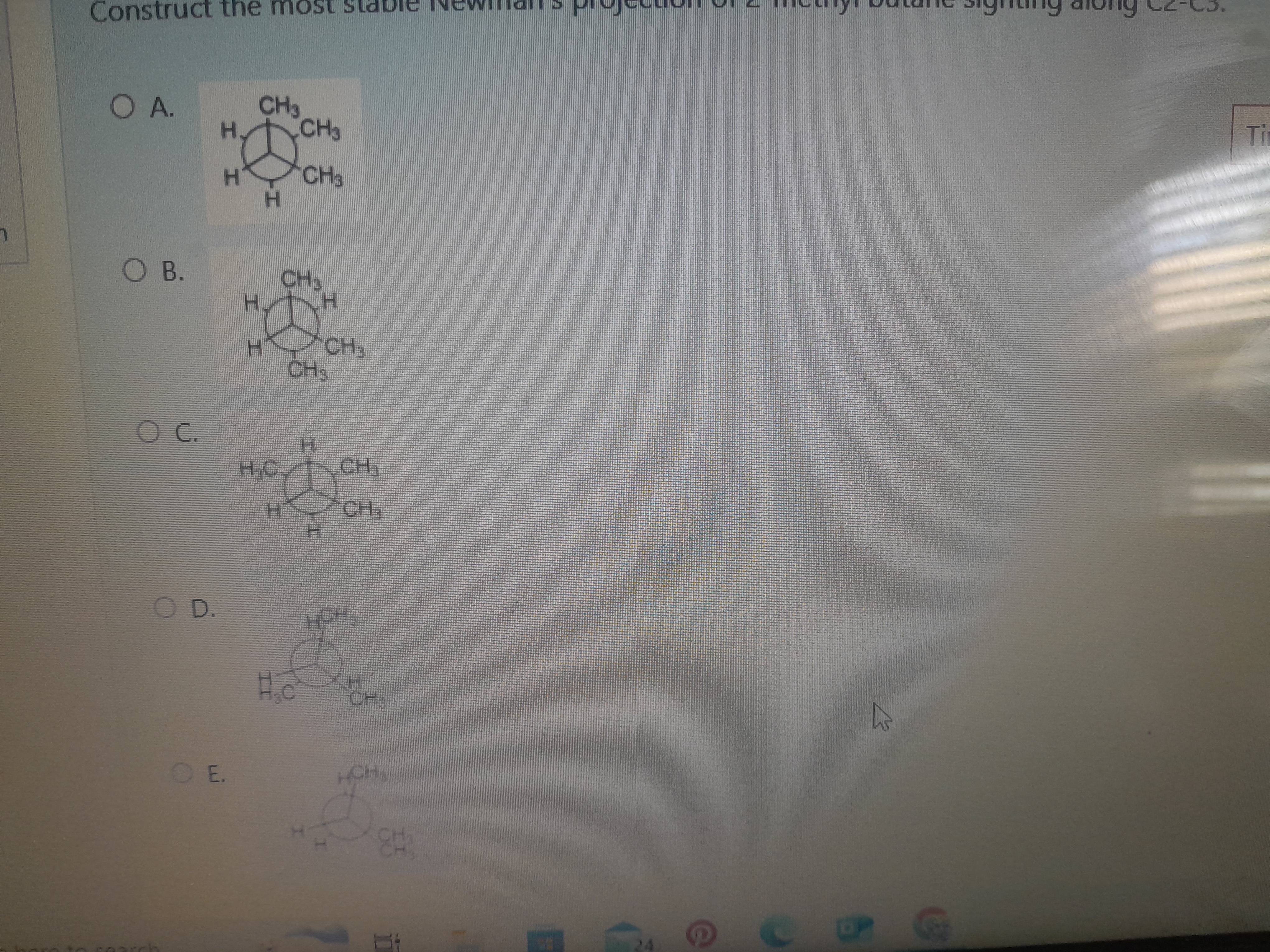
Construct the most stable Newman’s projection of 2-methylbutane viewing along C2-C3.
Understand the Problem
The question is asking to construct the most stable Newman projection of 2-methylbutane when viewing along the C2-C3 bond. This involves considering the spatial arrangement of atoms and how to minimize steric hindrance in the molecular structure.
Answer
Option C
The most stable Newman projection of 2-methylbutane is option C.
Answer for screen readers
The most stable Newman projection of 2-methylbutane is option C.
More Information
In the most stable conformation, large substituents are staggered to minimize steric hindrance, avoiding eclipsed interactions.
Tips
A common mistake is to overlook all relevant substituents when evaluating steric hindrance.
Sources
- Sighting along the C2-C3 bond of 2-methylbutane - homework.study.com
- Solved: Draw the most stable Newman Projection - chegg.com
AI-generated content may contain errors. Please verify critical information