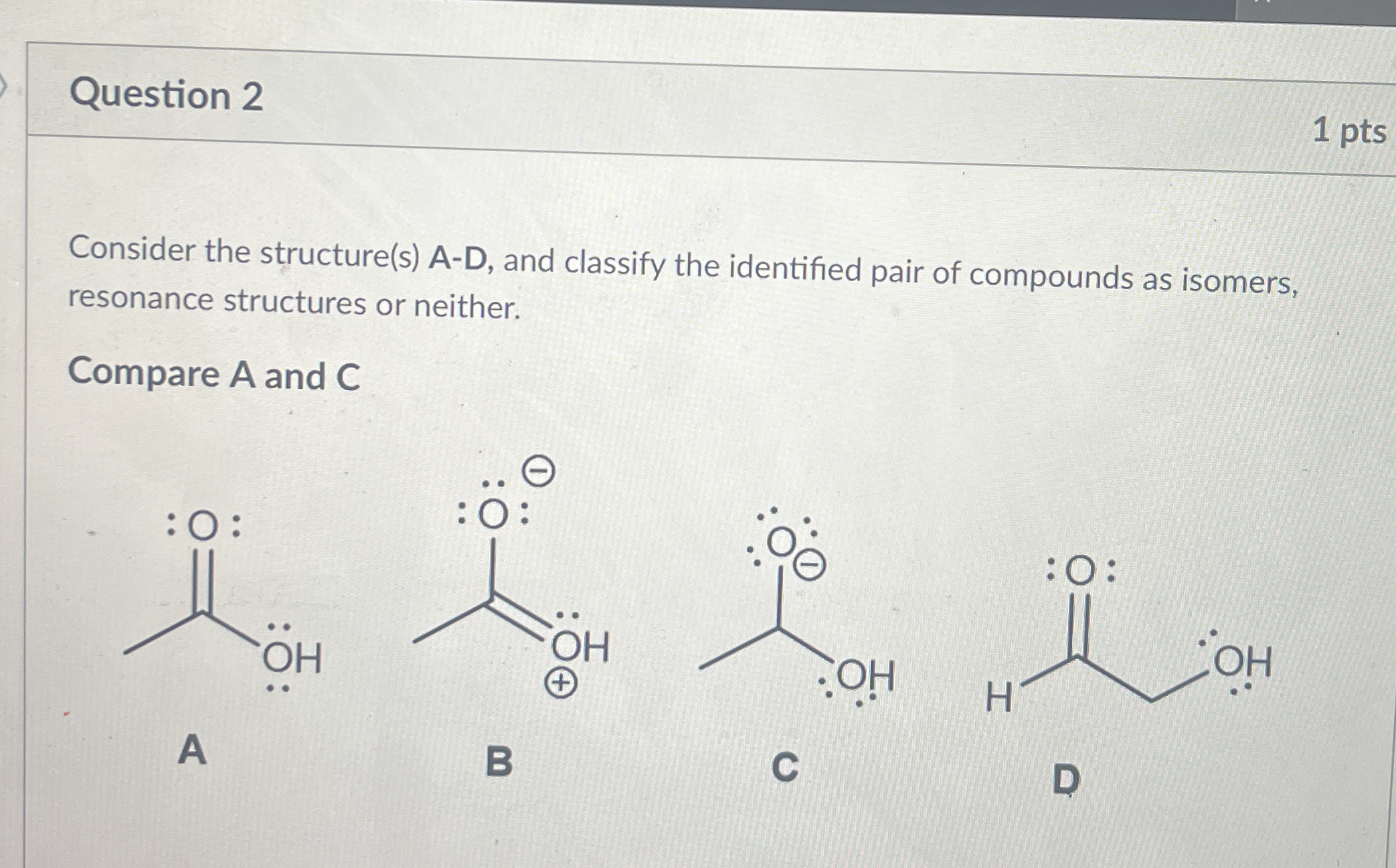
Consider the structure(s) A-D, and classify the identified pair of compounds as isomers, resonance structures or neither. Compare A and C.
Understand the Problem
The question is asking to compare two chemical structures (A and C) and classify them as isomers, resonance structures, or neither. This involves analyzing the molecular geometry and electronic structure of the compounds depicted.
Answer
Neither isomers nor resonance structures.
The structures A and C are neither isomers nor resonance structures.
Answer for screen readers
The structures A and C are neither isomers nor resonance structures.
More Information
Structure A is a simple carboxylic acid, while structure C is a resonance form of a carboxylate ion, indicating that they differ in atom connectivity and type.
Tips
A common mistake is to conflate resonance structures with distinct compounds; remember, resonance structures have the same connectivity and only differ in electron distribution.
AI-generated content may contain errors. Please verify critical information