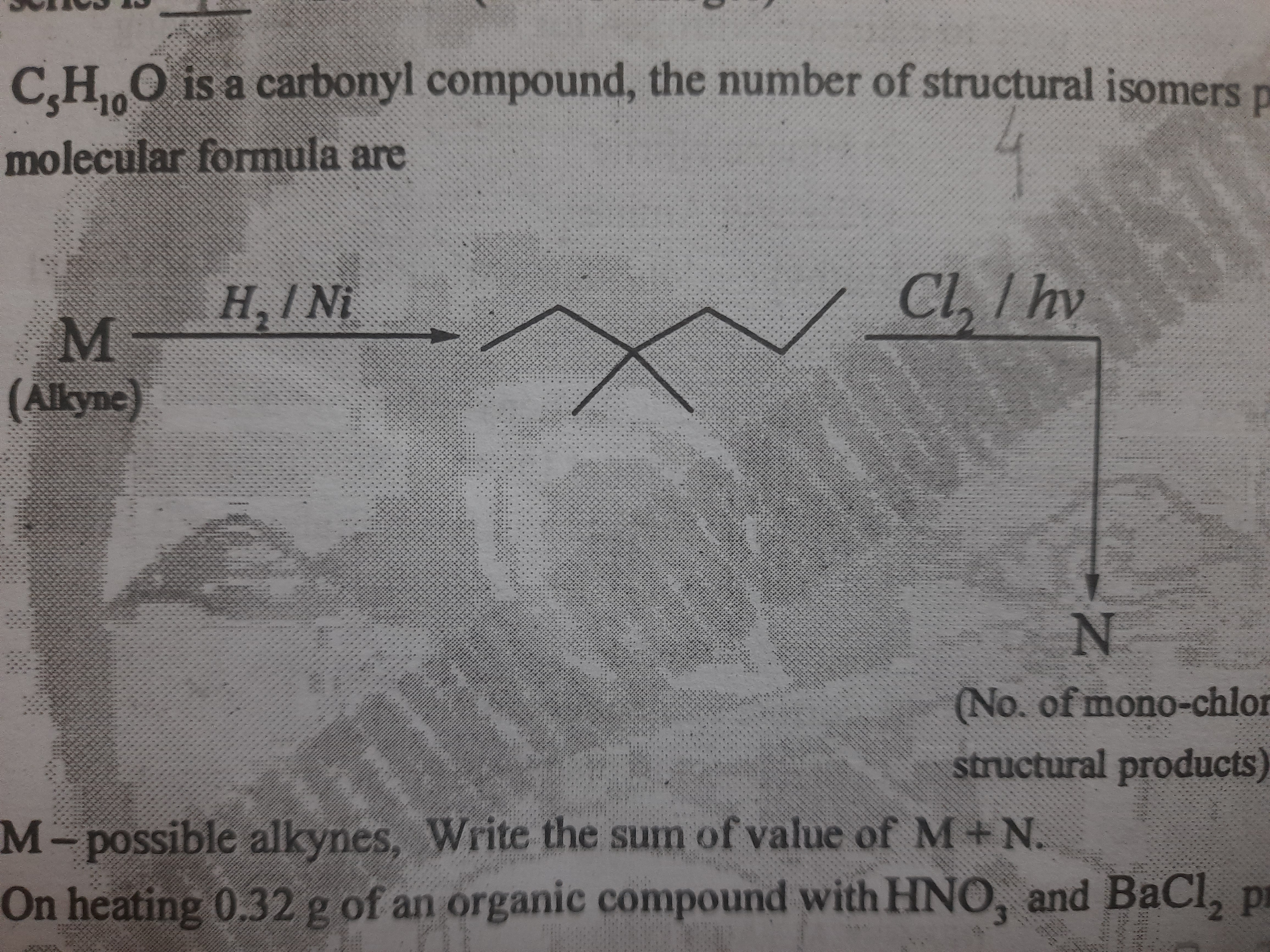
C5H10O is a carbonyl compound, the number of structural isomers are possible. M represents possible alkynes. Write the sum of the values of M and N. On heating 0.32 g of an organic... C5H10O is a carbonyl compound, the number of structural isomers are possible. M represents possible alkynes. Write the sum of the values of M and N. On heating 0.32 g of an organic compound with HNO3 and BaCl2, what are the results?
Understand the Problem
The question involves identifying possible alkynes (denoted as M) that can undergo certain reactions, calculating the number of structural isomers of a carbonyl compound with the molecular formula C5H10O, and the number of mono-chloro structural products (denoted as N). Finally, it asks for the sum of the values of M and N.
Answer
8
The sum of the values of M and N is 8.
Answer for screen readers
The sum of the values of M and N is 8.
More Information
The compound C5H10O can have 7 structural isomers as an aldehyde or a ketone. The number of possible alkynes with formula C5H8 is 3. Upon mono-chlorination of pentane, 3 monochloro products can be formed.
Tips
Ensure to correctly identify possible isomers by considering both aldehyde and ketone forms. For chlorination of alkanes, think about the different positions (primary, secondary, tertiary) where chlorine can substitute.
Sources
AI-generated content may contain errors. Please verify critical information