Understand the Problem
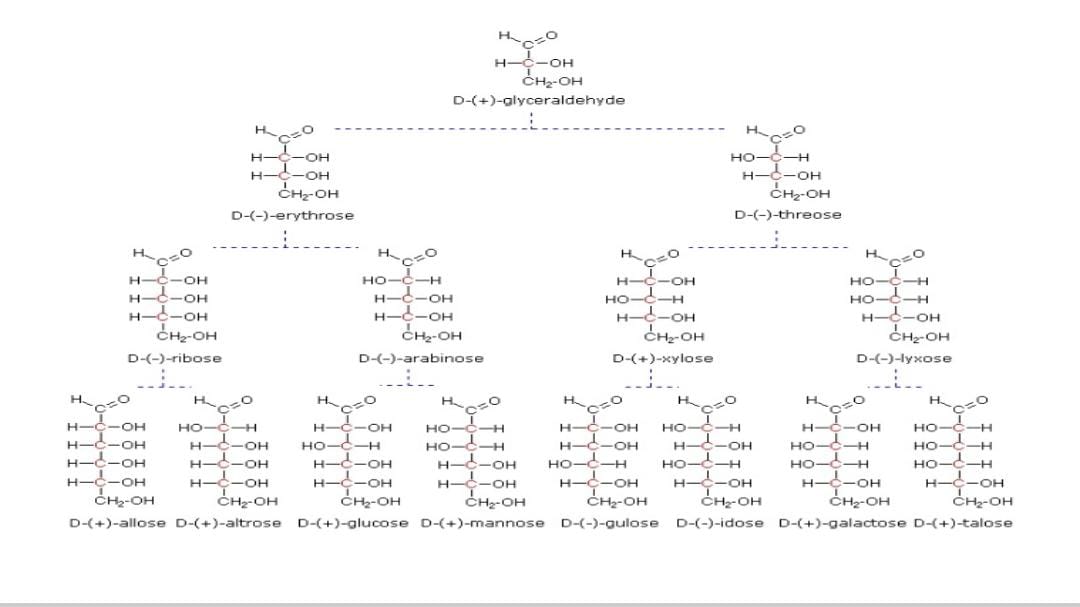
The image depicts a structural representation of various sugars and their interrelationships based on stereochemistry, specifically identifying D and L forms as well as different types of aldoses and ketoses.
Answer
The image illustrates the stereochemical evolution of sugars from D-glyceraldehyde.
The image shows the evolutionary progression of sugar molecules starting from D-(+)-glyceraldehyde, a simple sugar. It branches into more complex sugars, showing their structural arrangements through changes in the hydroxyl groups (-OH) orientation. This illustrates the stereochemistry of sugars.
Answer for screen readers
The image shows the evolutionary progression of sugar molecules starting from D-(+)-glyceraldehyde, a simple sugar. It branches into more complex sugars, showing their structural arrangements through changes in the hydroxyl groups (-OH) orientation. This illustrates the stereochemistry of sugars.
More Information
This image portrays a classic example of the Fischer projection used to represent the stereochemistry of sugars. Different rotations of the hydroxyl groups lead to various sugar isomers.
Tips
Not recognizing the stereochemistry may lead to confusion in identifying sugar types.
AI-generated content may contain errors. Please verify critical information