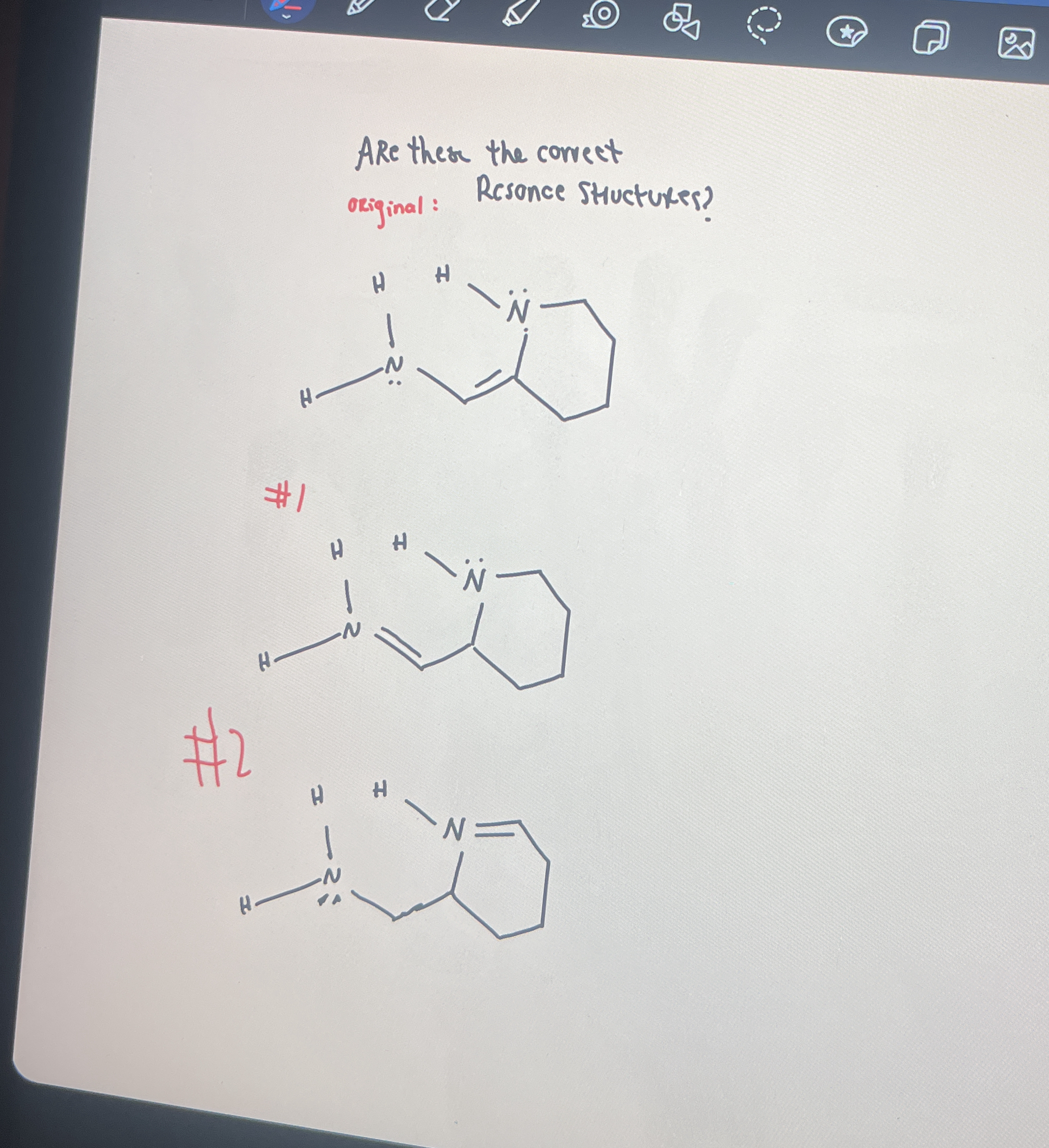
Are these the correct resonance structures?
Understand the Problem
The question is asking whether the provided structures are correct resonance structures based on the original structure shown. This involves assessing the validity of the two resonance forms drawn in the image.
Answer
Yes, the structures are correct resonance forms.
Both structures #1 and #2 are valid resonance structures for the original compound. They follow the rules for resonance, including valid Lewis structures and correct electron arrangement.
Answer for screen readers
Both structures #1 and #2 are valid resonance structures for the original compound. They follow the rules for resonance, including valid Lewis structures and correct electron arrangement.
More Information
Resonance structures are essential in understanding the delocalization of electrons in a molecule, which can affect the stability and reactivity of the compound.
Tips
Avoid drawing resonance structures that alter the positions of atoms or break the octet rule without justification.
Sources
- 2.4: Rules for Resonance Forms - Chemistry LibreTexts - chem.libretexts.org
AI-generated content may contain errors. Please verify critical information