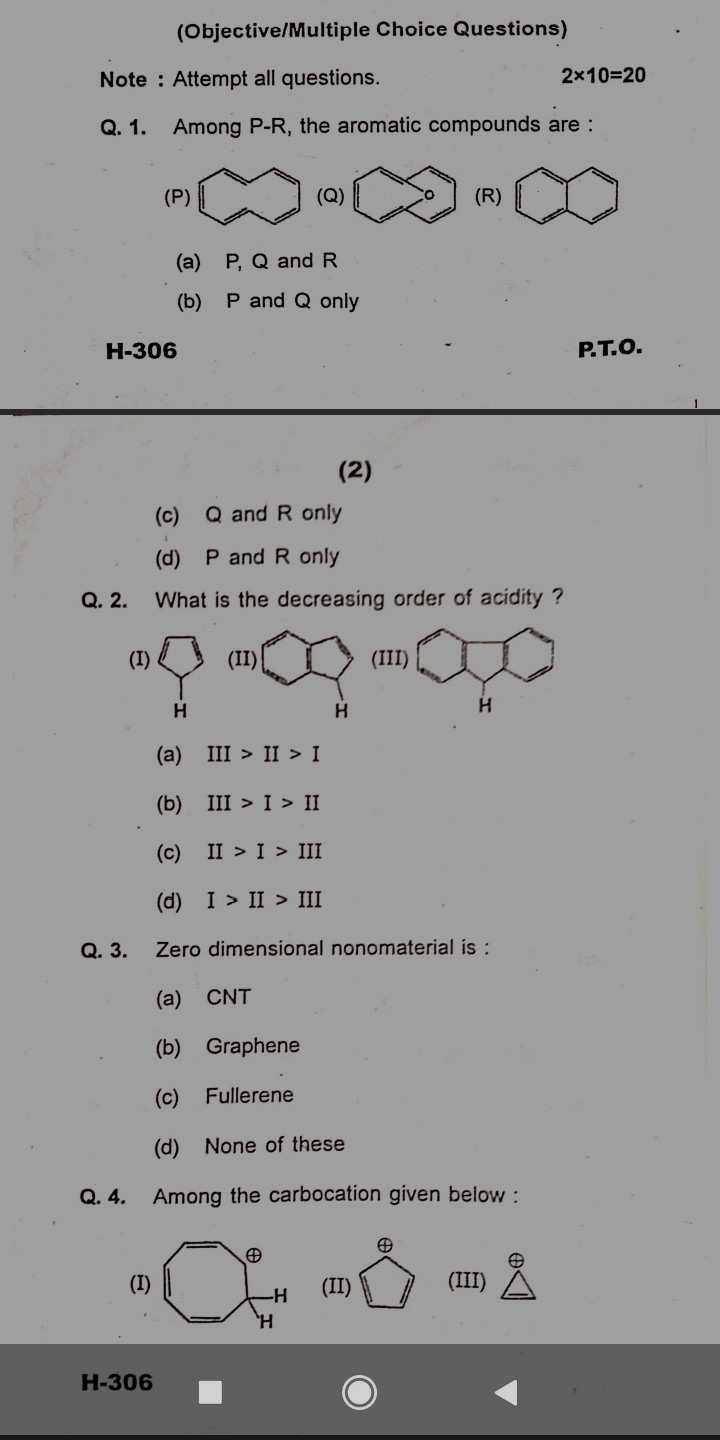
Among P-R, the aromatic compounds are: (P), (Q), (R). What is the decreasing order of acidity among the given structures? Zero dimensional nonomaterial is: CNT, Graphene, Fullerene... Among P-R, the aromatic compounds are: (P), (Q), (R). What is the decreasing order of acidity among the given structures? Zero dimensional nonomaterial is: CNT, Graphene, Fullerene, None of these. Among the carbocation given below: (I), (II), (III).
Understand the Problem
The question involves identifying the aromatic compounds among given structures and determining the decreasing order of acidity among several aromatic compounds. Additionally, it asks about zero-dimensional nonmaterial and examines carbocations.
Answer
1. Q and R. 2. III > II > I. 3. Fullerene. 4. (II).
- The aromatic compounds are Q and R. 2. The decreasing order of acidity is III > II > I. 3. Zero-dimensional nanomaterial is Fullerene. 4. The most stable carbocation among the ones given is (II).
Answer for screen readers
- The aromatic compounds are Q and R. 2. The decreasing order of acidity is III > II > I. 3. Zero-dimensional nanomaterial is Fullerene. 4. The most stable carbocation among the ones given is (II).
More Information
Aromatic compounds are determined by Huckel's rule, acidity by electron withdrawal, and nanomaterial dimensions by the confinement of electrons.
Tips
Ensure you apply Huckel's rule correctly for aromaticity, and consider resonance for acidity and carbocation stability.
AI-generated content may contain errors. Please verify critical information