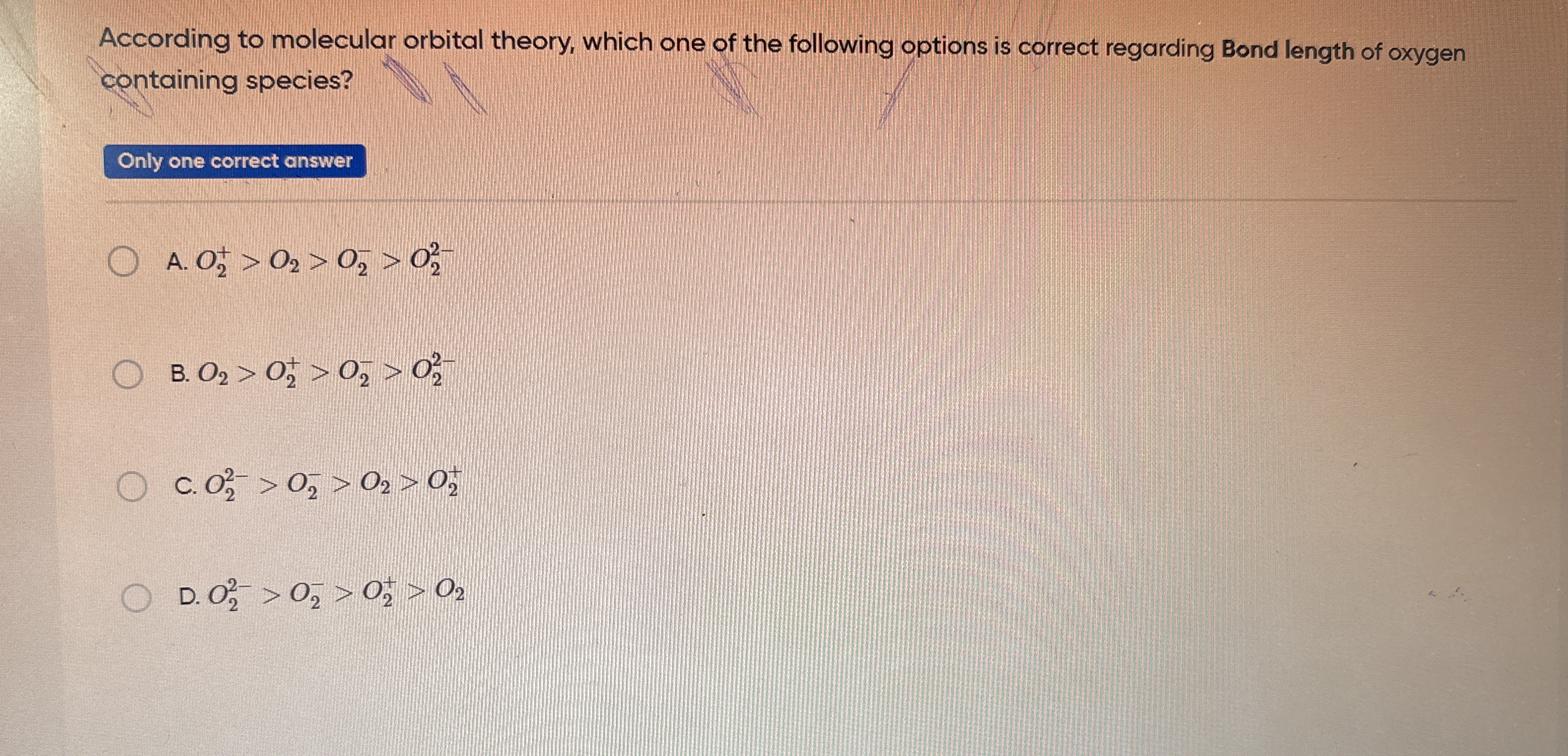
According to molecular orbital theory, which one of the following options is correct regarding bond length of oxygen containing species?
Understand the Problem
The question is asking to determine the correct order of bond lengths for various oxygen-containing species based on molecular orbital theory. This involves comparing the bond lengths of ions and molecules like O2, O2+, and O22-.
Answer
O2 2- > O2 - > O2 > O2 +
The correct order regarding bond length, from longest to shortest, is O2 2- > O2 - > O2 > O2 +.
Answer for screen readers
The correct order regarding bond length, from longest to shortest, is O2 2- > O2 - > O2 > O2 +.
More Information
Molecular orbital theory describes how bond order influences bond length. Higher bond order means shorter bond length due to stronger bonding interactions.
Tips
Common mistakes include miscalculating bond order and forgetting the inverse relationship between bond length and bond order.
Sources
AI-generated content may contain errors. Please verify critical information