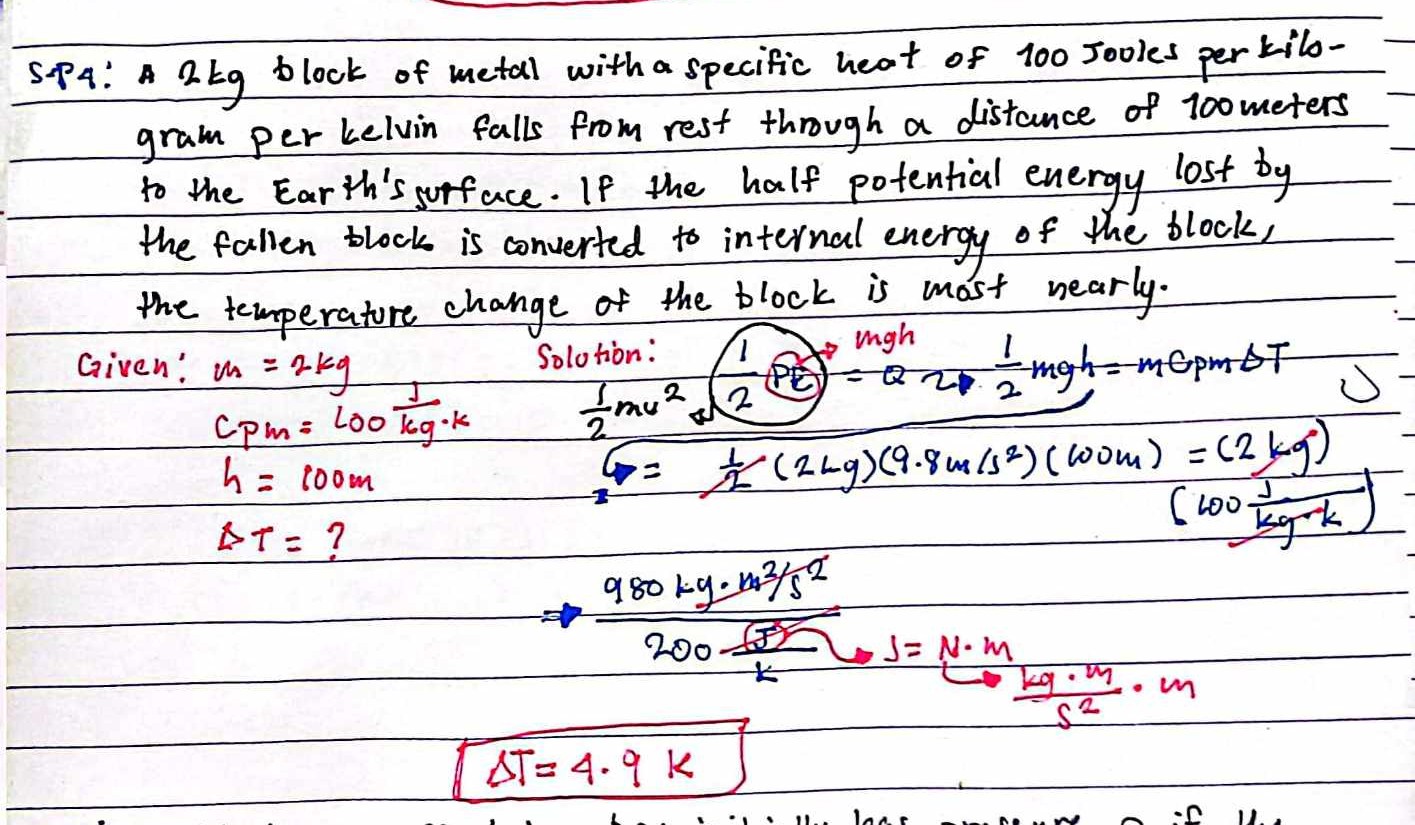
A 2 kg block of metal with a specific heat of 100 Joules per kilogram per Kelvin falls from rest through a distance of 100 meters to the Earth's surface. If the half potential ener... A 2 kg block of metal with a specific heat of 100 Joules per kilogram per Kelvin falls from rest through a distance of 100 meters to the Earth's surface. If the half potential energy lost by the fallen block is converted to internal energy of the block, the temperature change of the block is most nearly.
Understand the Problem
The question is asking to determine the temperature change of a 2 kg block of metal that falls a distance of 100 meters, where half of its potential energy is converted to internal energy. We need to calculate the temperature rise based on the specific heat of the metal.
Answer
The temperature change is \( \Delta T = 4.9 \, \text{K} \).
Answer for screen readers
The temperature change of the block is ( \Delta T = 4.9 , \text{K} ).
Steps to Solve
- Calculate Potential Energy (PE)
The formula for potential energy is given by:
$$ PE = mgh $$
Where:
- ( m = 2 , \text{kg} ) (mass)
- ( g = 9.8 , \text{m/s}^2 ) (acceleration due to gravity)
- ( h = 100 , \text{m} ) (height)
Plugging in the values:
$$ PE = 2 \times 9.8 \times 100 = 1960 , \text{J} $$
- Determine Internal Energy Converted
According to the problem, half of the potential energy is converted to internal energy:
$$ Q = \frac{1}{2} PE = \frac{1}{2} \times 1960 = 980 , \text{J} $$
- Relate Internal Energy to Temperature Change
The formula relating heat (internal energy) to temperature change is:
$$ Q = mc_{p} \Delta T $$
Where:
- ( Q = 980 , \text{J} )
- ( m = 2 , \text{kg} )
- ( c_{p} = 100 , \text{J/(kg \cdot K)} )
- Solve for Temperature Change (ΔT)
Rearranging the equation gives:
$$ \Delta T = \frac{Q}{mc_p} $$
Now plug in the values:
$$ \Delta T = \frac{980}{2 \times 100} = \frac{980}{200} = 4.9 , \text{K} $$
The temperature change of the block is ( \Delta T = 4.9 , \text{K} ).
More Information
This calculation demonstrates how potential energy converts into internal energy when an object falls. The specific heat capacity ( c_p ) of the metal plays an essential role in determining the temperature rise of the block.
Tips
- Forgetting to take half of the potential energy into account.
- Using incorrect units for specific heat or mass, which can lead to calculation errors.
- Misapplying the gravitational acceleration, assuming it varies with height for small distances.
AI-generated content may contain errors. Please verify critical information