78.084 g/mL × 47 mL = ? g
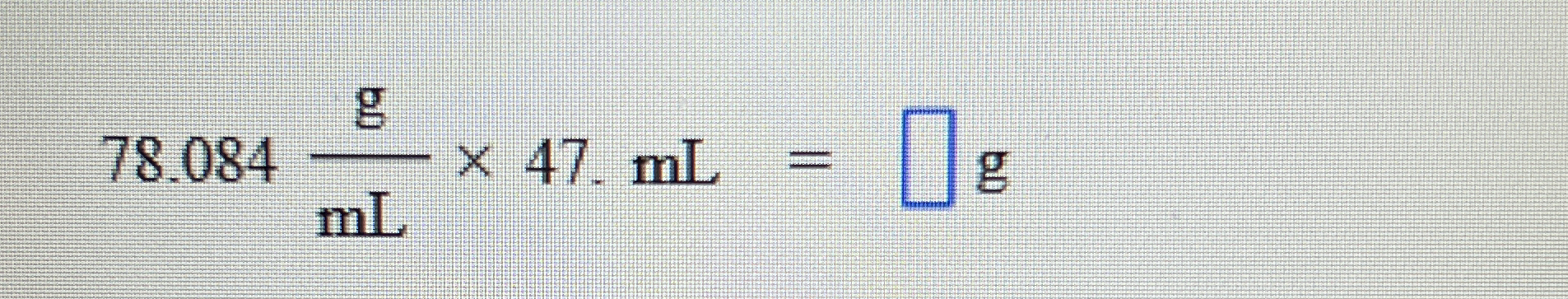
Understand the Problem
The question is asking us to calculate the mass in grams using the provided density (78.084 g/mL) and volume (47 mL). To solve this, we will multiply the density by the volume to find the mass.
Answer
The mass is approximately $3660.948$ g.
Answer for screen readers
The mass is approximately 3660.948 g.
Steps to Solve
- Multiply Density by Volume
To find the mass, we multiply the density by the volume. Here, the formula to use is:
$$ \text{Mass} = \text{Density} \times \text{Volume} $$
Substituting the given values:
$$ \text{Mass} = 78.084 , \text{g/mL} \times 47 , \text{mL} $$
- Perform the Calculation
Now, perform the multiplication:
[ \text{Mass} = 78.084 \times 47 ]
Calculating this gives:
$$ \text{Mass} = 3660.948 , \text{g} $$
- Round the Result
Since we generally report density measurements to three decimal places, we keep our result to three decimal places as well:
$$ \text{Mass} \approx 3660.948 , \text{g} $$
The mass is approximately 3660.948 g.
More Information
This calculation shows how to find mass using the relationship between density, volume, and mass. Density helps determine how much matter exists in a given volume, and knowing the density allows for easy mass calculation using the provided volume.
Tips
- Not using correct units: Ensure that the units of density and volume are compatible when performing the multiplication.
- Forgetting to multiply correctly: Careful with the numbers to avoid arithmetic errors.
AI-generated content may contain errors. Please verify critical information