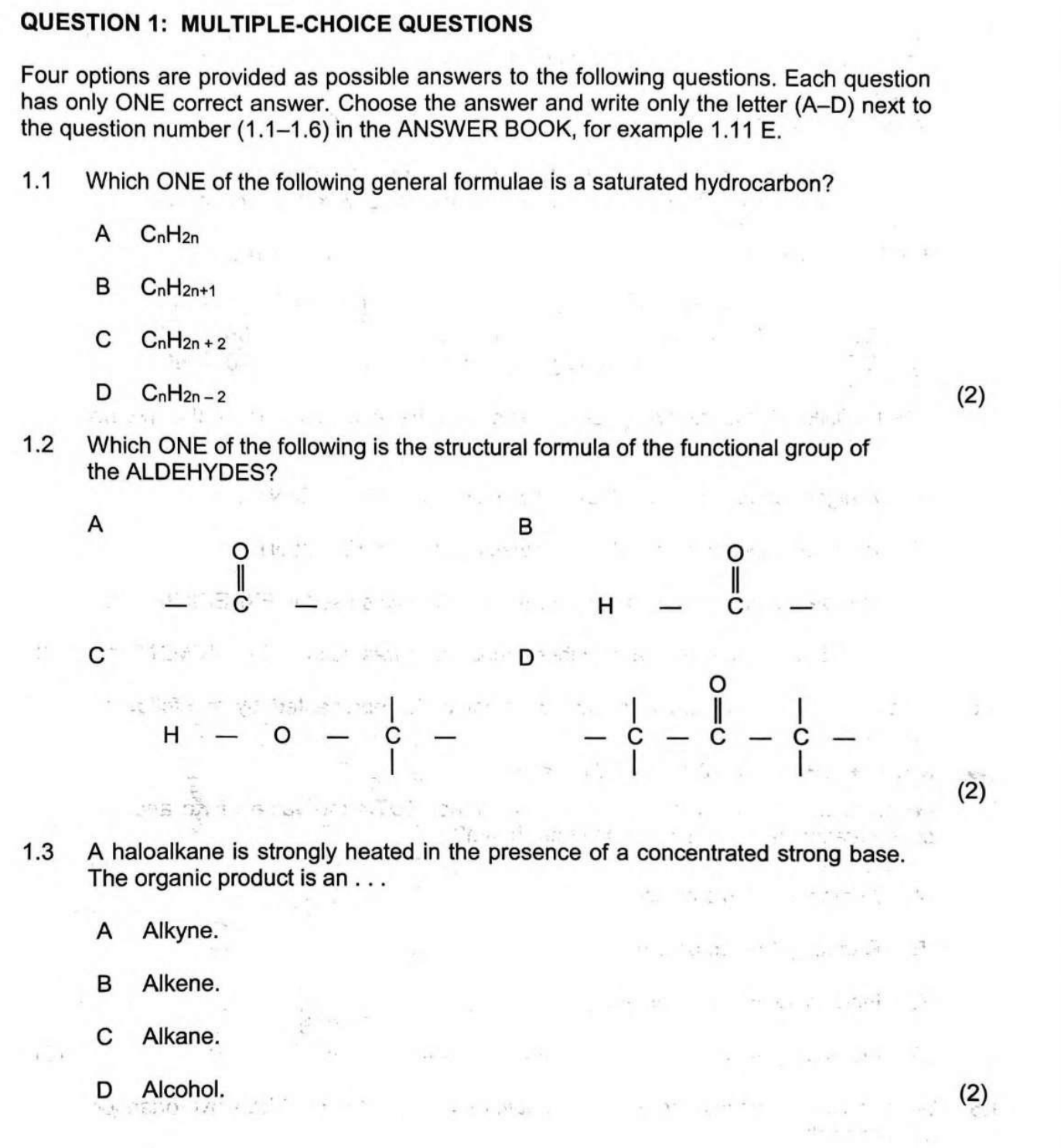
1.1 Which ONE of the following general formulae is a saturated hydrocarbon? A CnH2n B CnH2n+1 C CnH2n+2 D CnH2n-2 1.2 Which ONE of the following is the structural formula of the fu... 1.1 Which ONE of the following general formulae is a saturated hydrocarbon? A CnH2n B CnH2n+1 C CnH2n+2 D CnH2n-2 1.2 Which ONE of the following is the structural formula of the functional group of the ALDEHYDES? A O || C - H B O || C C H - O - C - C D O || C - C - C 1.3 A haloalkane is strongly heated in the presence of a concentrated strong base. The organic product is an ... A Alkyne B Alkene C Alkane D Alcohol.
Understand the Problem
The question is asking about multiple-choice questions related to organic chemistry, specifically focusing on saturated hydrocarbons, structural formulas of aldehydes, and the products of reactions involving haloalkanes. Each part of the question requires identifying correct answers from the provided options.
Answer
1.1: CnH2n+2 (C), 1.2: O || C - H (B), 1.3: Alkene (B)
1.1: CnH2n+2 (C), 1.2: O || C - H (B), 1.3: Alkene (B)
Answer for screen readers
1.1: CnH2n+2 (C), 1.2: O || C - H (B), 1.3: Alkene (B)
More Information
Saturated hydrocarbons are alkanes, which follow the general formula CnH2n+2. Aldehydes have the functional group O=C-H. Heating haloalkanes with a strong base typically results in an elimination reaction, forming an alkene.
Tips
A common mistake is confusing the functional groups for aldehydes and ketones. Aldehydes have the carbonyl group (C=O) at the end of a carbon chain, unlike ketones.