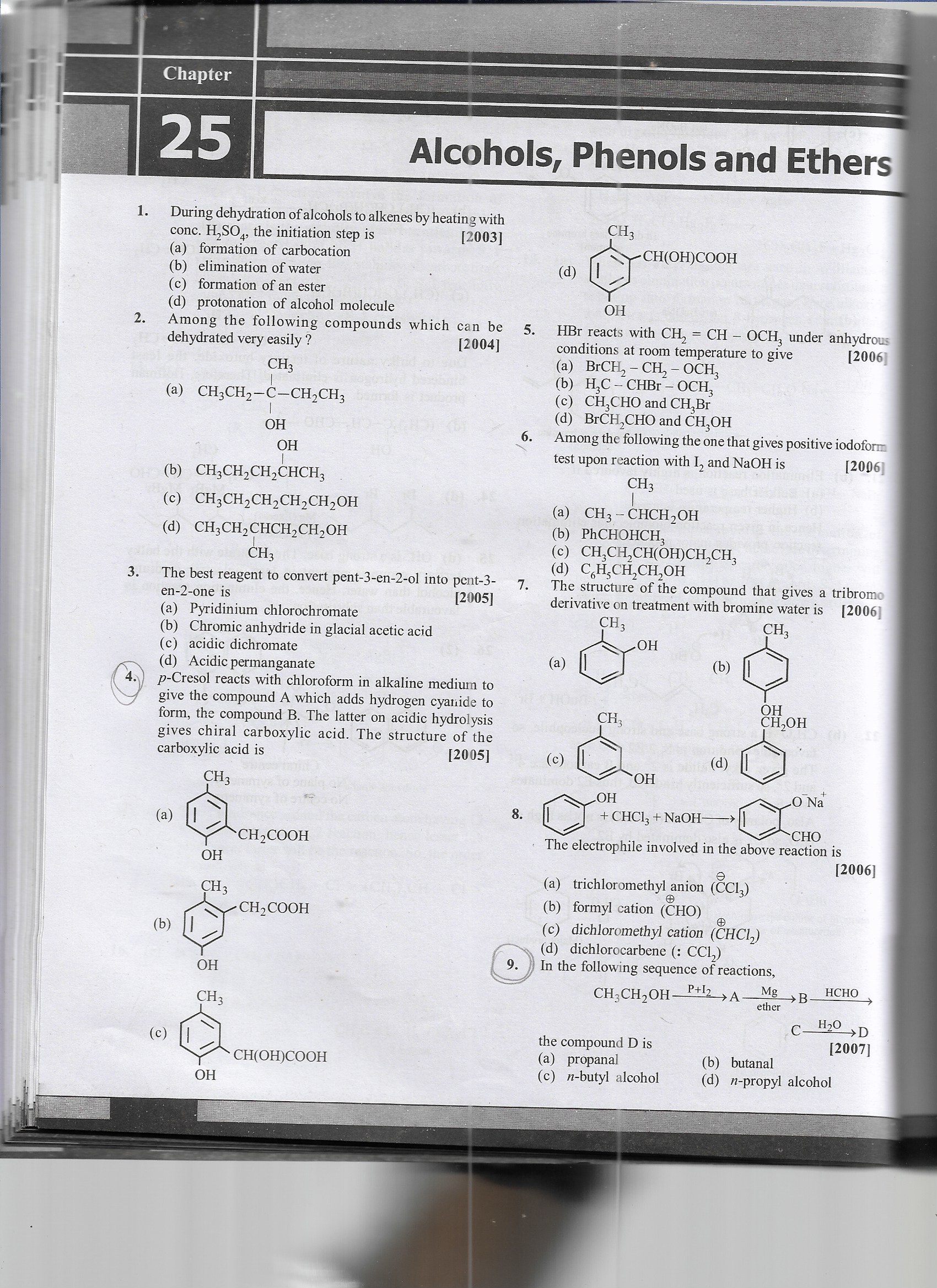
1. During dehydration of alcohols to alkenes by heating with conc. H2SO4, the initiation step is (a) formation of carbocation (b) elimination of water (c) formation of an ester (d)... 1. During dehydration of alcohols to alkenes by heating with conc. H2SO4, the initiation step is (a) formation of carbocation (b) elimination of water (c) formation of an ester (d) protonation of alcohol molecule? 2. Among the following compounds which can be dehydrated very easily? (a) CH3CH2-C-CH2CH3 (b) CH3CH2CH2CH3 (c) CH3CH2CH2CH2OH (d) CH3CH2CHCH2OH 3. The best reagent to convert pent-3-en-2-ol into pent-3-en-2-one is (a) Pyridinium chlorochromate (b) Chromic anhydride in glacial acetic acid (c) acidic dichromate (d) Acidic permanganate 4. p-Cresol reacts with chloroform in alkaline medium to give the compound A which adds hydrogen cyanide to form, the compound B. The latter on acidic hydrolysis gives chiral carboxylic acid. The structure of the carboxylic acid is (a) CH3C(=O)OH (b) CH3C(=O)OH (c) CH3C(=O)OH (d) CH3C(=O)OH 5. HBr reacts with CH2=CH-OCH3 under anhydrous conditions at room temperature to give (a) BrCH2-CH-OCH3 (b) H2C=CHBr-OCH3 (c) CH3CHO and CH3Br (d) BrCH2CHO and CH3OH 6. Among the following the one that gives positive iodoform test upon reaction with I2 and NaOH is (a) CH3-CHCH2OH (b) P(CHOH)CH3 (c) CH3CH(OH)CH3 (d) CH3CH2CH2OH 7. The structure of the compound that gives a tribromo derivative on treatment with bromine water is (a) CH3 (b) (c) (d) 8. The electrophile involved in the above reaction is (a) trichloromethyl anion (b) formyl cation (c) dichloromethyl cation (d) dichlorocarbene (CCl2) 9. In the following sequence of reactions, CH3CH2OH + PBr3 → A + Mg → B + H2O → D the compound D is (a) propanal (b) n-butyl alcohol (c) n-propyl alcohol?
Understand the Problem
The question consists of various chemistry problems related to the topic of alcohols, phenols, and ethers. It seeks to assess knowledge on dehydration of alcohols, reagents in reactions, and structural analysis of compounds. Each query includes multiple-choice options and is designed to test chemical understanding and application.