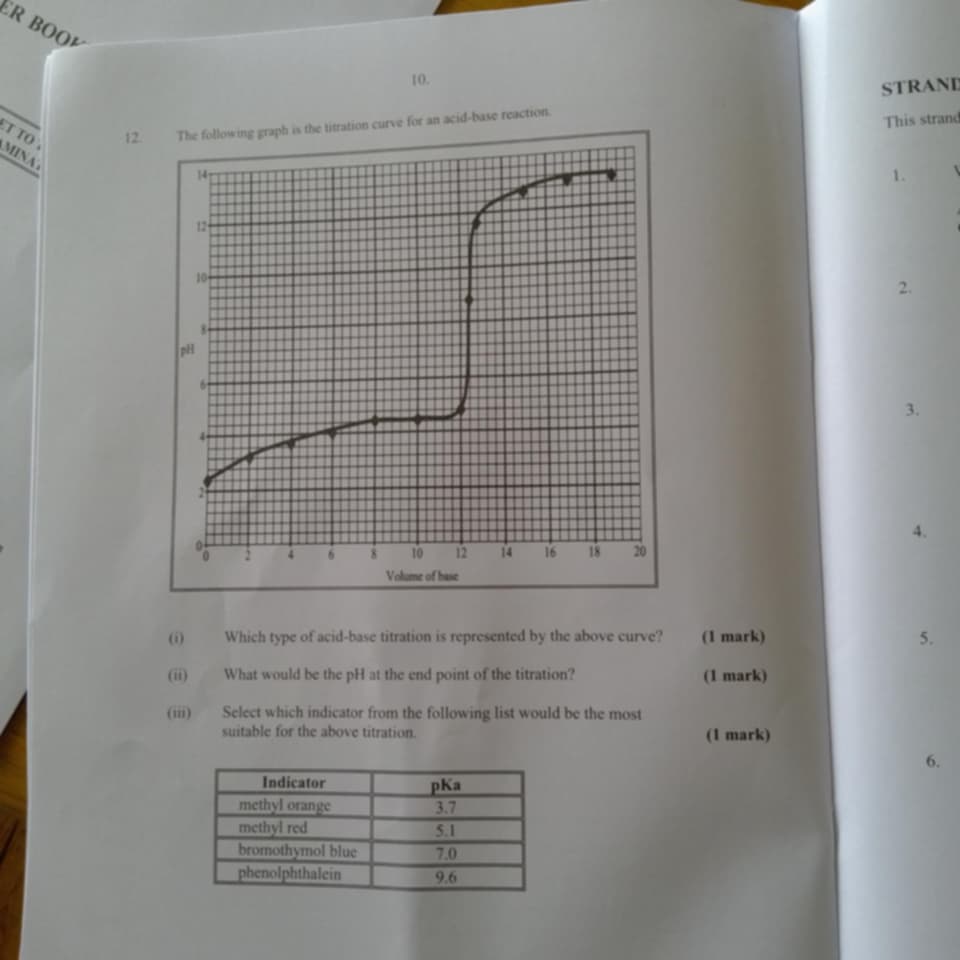
Which type of acid-base titration is represented by the above curve? What would be the pH at the end point of the titration? Select which indicator from the following list would be... Which type of acid-base titration is represented by the above curve? What would be the pH at the end point of the titration? Select which indicator from the following list would be the most suitable for the above titration.
Understand the Problem
The question is asking about an acid-base titration curve, specifically what type of titration it represents, the pH at the endpoint, and which indicator would be most suitable based on given pKa values.
Answer
Weak acid-strong base titration; pH ~9; Phenolphthalein.
The titration curve represents a weak acid with a strong base titration. The pH at the endpoint is above 7, likely around 9. Phenolphthalein is the most suitable indicator.
Answer for screen readers
The titration curve represents a weak acid with a strong base titration. The pH at the endpoint is above 7, likely around 9. Phenolphthalein is the most suitable indicator.
More Information
In a weak acid-strong base titration, the equivalence point is often above pH 7 due to the formation of a basic salt. Phenolphthalein, changing color in the pH range of 8 to 10, is appropriate.
Tips
A common mistake is choosing an indicator without considering the pH range in relation to the equivalence point.
Sources
- Titration curves & equivalence point - khanacademy.org
- Acid Base Titration - byjus.com
- Acid-Base Indicators - knowledge.carolina.com
AI-generated content may contain errors. Please verify critical information