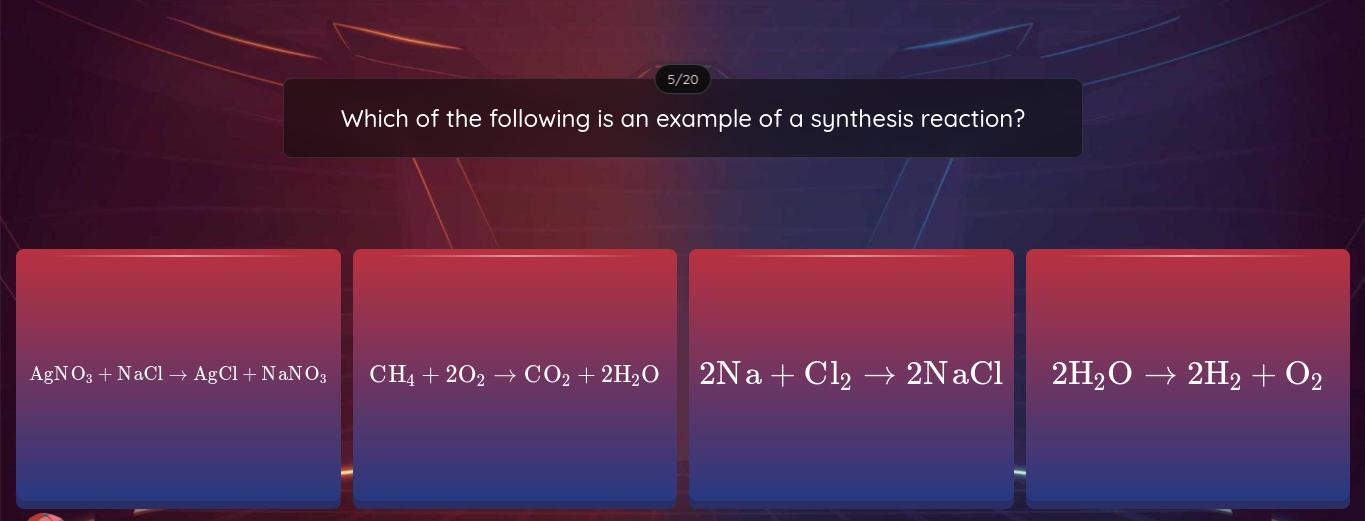
Which of the following is an example of a synthesis reaction?
Understand the Problem
The question is asking which of the provided chemical equations represents a synthesis reaction, where two or more substances combine to form a single product.
Answer
2Na + Cl₂ → 2NaCl
The reaction 2Na + Cl₂ → 2NaCl is an example of a synthesis reaction.
Answer for screen readers
The reaction 2Na + Cl₂ → 2NaCl is an example of a synthesis reaction.
More Information
A synthesis reaction is a type of chemical reaction in which simple compounds combine to form a more complex compound. It is exothermic, often releasing energy.
Tips
Common mistake is confusing synthesis reactions with decomposition or combustion reactions. Remember, synthesis reactions yield one product from multiple reactants.
Sources
- Synthesis Reaction | CK-12 Foundation - flexbooks.ck12.org
AI-generated content may contain errors. Please verify critical information