Which of the following decreases while moving left to right on the periodic table?
Understand the Problem
The question asks which property decreases when moving from left to right on the periodic table. It provides multiple-choice options related to atomic properties, and the user is trying to identify the correct one based on their knowledge of periodic trends.
Answer
Atomic radius
The final answer is atomic radius.
Answer for screen readers
The final answer is atomic radius.
More Information
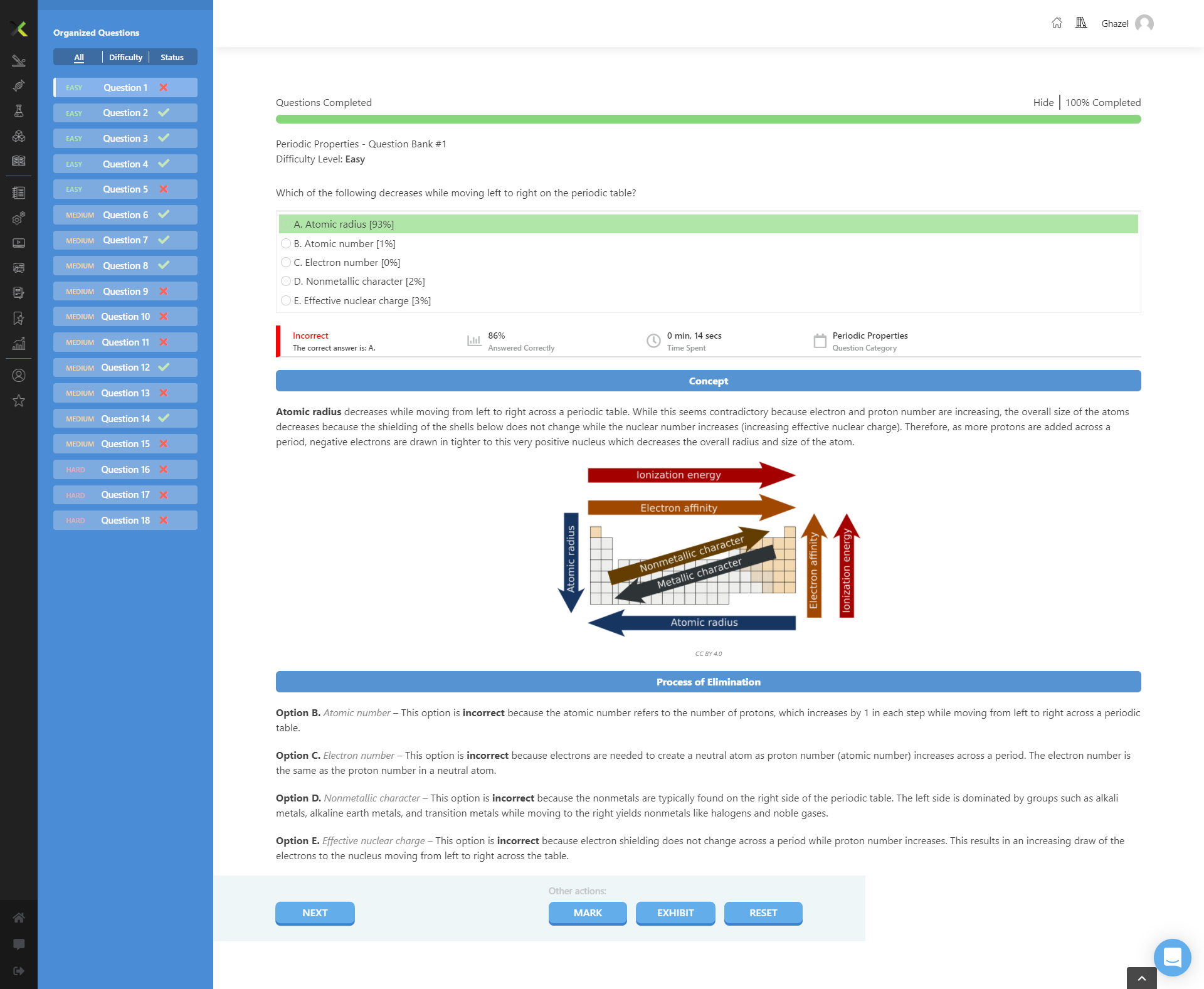
As you move from left to right across a period on the periodic table, the atomic radius decreases due to the increase in nuclear charge which pulls electrons closer to the nucleus.
Tips
Common mistakes include confusing atomic radius with electron number, which actually increases across a period.
Sources
AI-generated content may contain errors. Please verify critical information