Which of the following decreases while moving left to right on the periodic table?
Understand the Problem
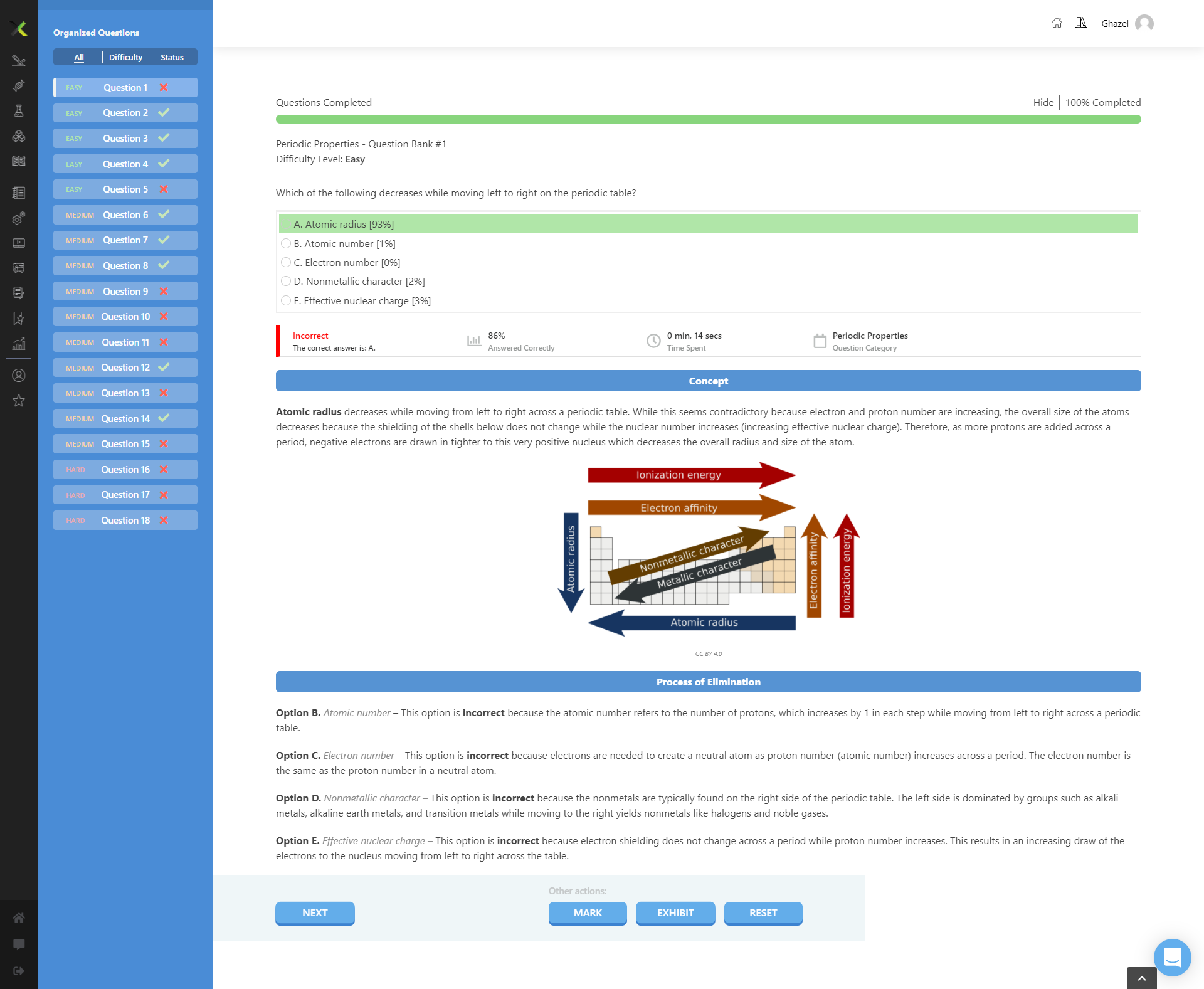
The question is asking which property decreases when moving from left to right in the periodic table. It presents multiple options related to properties of elements in the periodic table and expects the identification of the correct decreasing property.
Answer
Atomic radius
The final answer is atomic radius.
Answer for screen readers
The final answer is atomic radius.
More Information
As you move from left to right on the periodic table, atomic size decreases due to increased nuclear charge attracting the valence electrons closer, reducing atomic radius.
Tips
Remember that while proton number increases across a period, causing greater nuclear attraction, the number of electron shells remains constant, resulting in a smaller atomic radius.
Sources
- Atomic size trend - BYJU's - byjus.com
- Atomic radius decreases across the period - Toppr - toppr.com
AI-generated content may contain errors. Please verify critical information