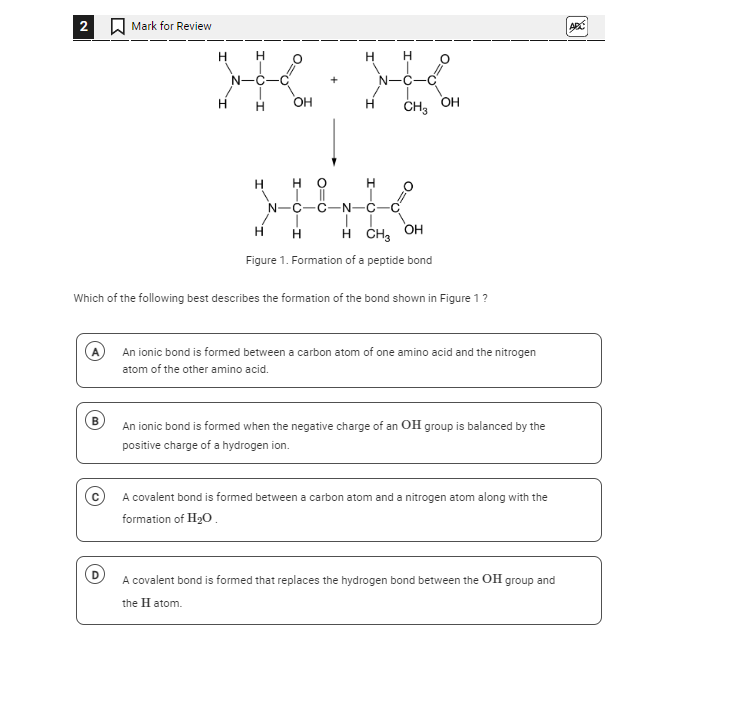
Which of the following best describes the formation of the bond shown in Figure 1?
Understand the Problem
The question is asking which option correctly describes the formation of a peptide bond as illustrated in Figure 1. The goal is to understand the type of bond created when amino acids combine through dehydration synthesis.
Answer
A covalent bond is formed between a carbon atom and a nitrogen atom along with the formation of H₂O.
A covalent bond is formed between a carbon atom and a nitrogen atom along with the formation of H₂O.
Answer for screen readers
A covalent bond is formed between a carbon atom and a nitrogen atom along with the formation of H₂O.
More Information
The bond shown in Figure 1 is a peptide bond, which is formed by a dehydration synthesis reaction where a covalent bond forms between the carboxyl group of one amino acid and the amine group of another, with water being released as a byproduct.
Tips
A common mistake is to confuse peptide bonds with ionic bonds. Peptide bonds are covalent and occur between amino acids in proteins.
Sources
- Biochemistry Flashcards by Lily Jones | Brainscape - brainscape.com
- Organic vs ( Inorganic Compounds - Course Sidekick - coursesidekick.com
- AP Bio - How Macromolecules Are Joined and Broken | Quizizz - quizizz.com
AI-generated content may contain errors. Please verify critical information