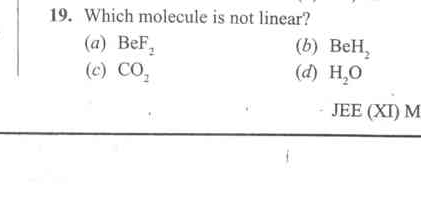
Which molecule is not linear? (a) BeF2 (b) BeH2 (c) CO2 (d) H2O
Understand the Problem
The question is asking to identify a molecule from a list that does not have a linear molecular geometry. The goal is to analyze the molecular structures of the given options.
Answer
H2O
The final answer is H2O, as it is not linear but bent due to sp3 hybridization.
Answer for screen readers
The final answer is H2O, as it is not linear but bent due to sp3 hybridization.
More Information
The water molecule (H2O) is bent due to the presence of two lone pairs of electrons on the oxygen atom, which causes a distortion and prevents a linear shape.
Tips
A common mistake is assuming that all triatomic molecules are linear. However, the presence of lone pairs can lead to a bent shape.
Sources
- Which one of the following molecules is not linear? - Doubtnut - doubtnut.com
- Which molecule is not linear? - BYJU'S - byjus.com
AI-generated content may contain errors. Please verify critical information