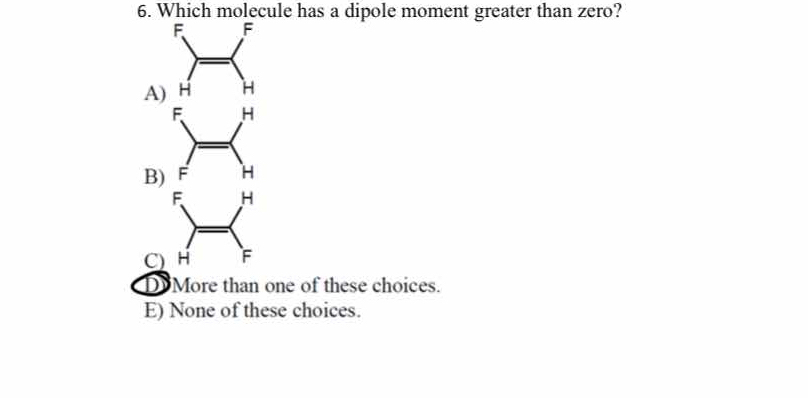
Which molecule has a dipole moment greater than zero?
Understand the Problem
The question is asking which of the provided molecular structures has a dipole moment that is greater than zero. This relates to the concept of molecular polarity in chemistry.
Answer
C) More than one of these choices.
The final answer is C) More than one of these choices.
Answer for screen readers
The final answer is C) More than one of these choices.
More Information
In the given options, both structures A and B have substitutions that do not entirely cancel out the dipole moments, suggesting more than one molecule with a non-zero dipole moment.
Tips
A common mistake is assuming symmetric-looking molecules automatically have zero dipole moment. Evaluate the vector sum of dipoles carefully.
AI-generated content may contain errors. Please verify critical information