Which compound would have the lowest boiling point?
Understand the Problem
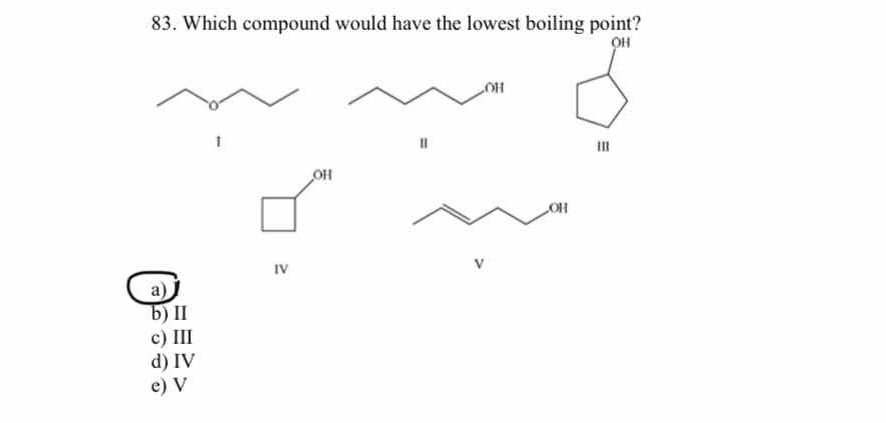
The question is asking which of the given chemical compounds has the lowest boiling point, which is influenced by factors such as molecular structure and intermolecular forces.
Answer
I
The final answer is I
Answer for screen readers
The final answer is I
More Information
Compound I, likely an ether, has the weakest intermolecular forces among the options because it primarily has dispersion forces whereas others have hydrogen bonding.
Tips
A common mistake is not recognizing the types of intermolecular forces present in each compound.
Sources
- The web page with info on - Example Source - wyzant.com
AI-generated content may contain errors. Please verify critical information