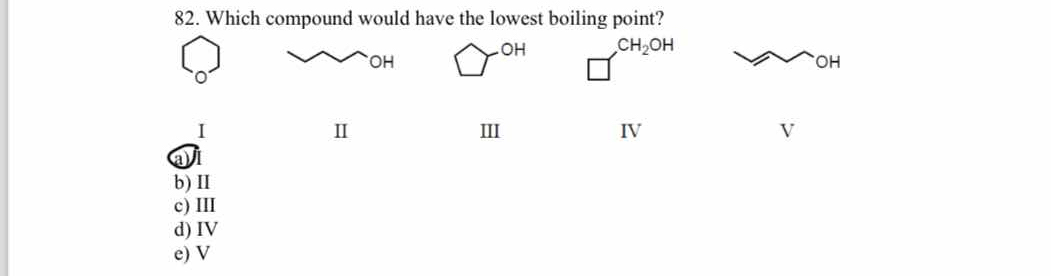
Which compound would have the lowest boiling point?
Understand the Problem
The question is asking which of the given compounds has the lowest boiling point. It requires an understanding of the factors that influence boiling points, such as molecular structure and intermolecular forces.
Answer
I
The final answer is I.
Answer for screen readers
The final answer is I.
More Information
Compound I is an ether with only dispersion forces, leading to a lower boiling point compared to the alcohols that can form hydrogen bonds.
Tips
A common mistake is not considering intermolecular forces like hydrogen bonding, which increases boiling points significantly in alcohols.
AI-generated content may contain errors. Please verify critical information