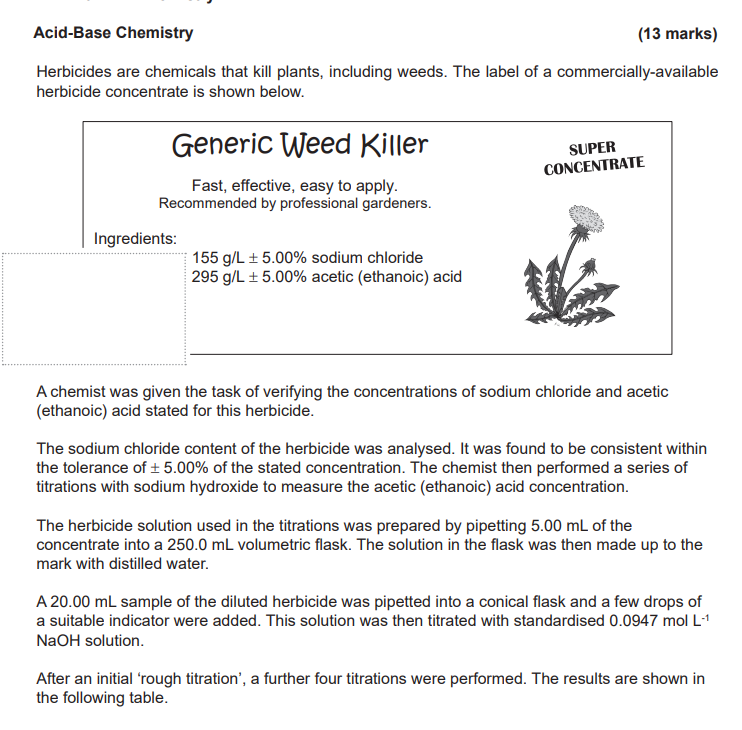
What were the results of the titrations performed by the chemist?
Understand the Problem
The question provides a scenario involving chemical titration used to verify the concentrations of sodium chloride and acetic acid in a herbicide. It seeks to gather information about the results of titrations conducted by the chemist.
Answer
Analyze titration results for acetic acid vs. 295 g/L ± 5%.
The results of the titrations need to be analyzed based on the table provided, which was not included. The concentrations measured should be compared to the labeled specification of 295 g/L ± 5% acetic acid concentration.
Answer for screen readers
The results of the titrations need to be analyzed based on the table provided, which was not included. The concentrations measured should be compared to the labeled specification of 295 g/L ± 5% acetic acid concentration.
More Information
To verify herbicide concentration, the chemist uses titrations with NaOH. The standardization involves a known concentration to determine the concentration of the acetic acid in the herbicide. The results help ensure product formulation meets labeling standards.
Tips
Common mistakes include miscalculating dilution factors or errors in volume measurements.
AI-generated content may contain errors. Please verify critical information