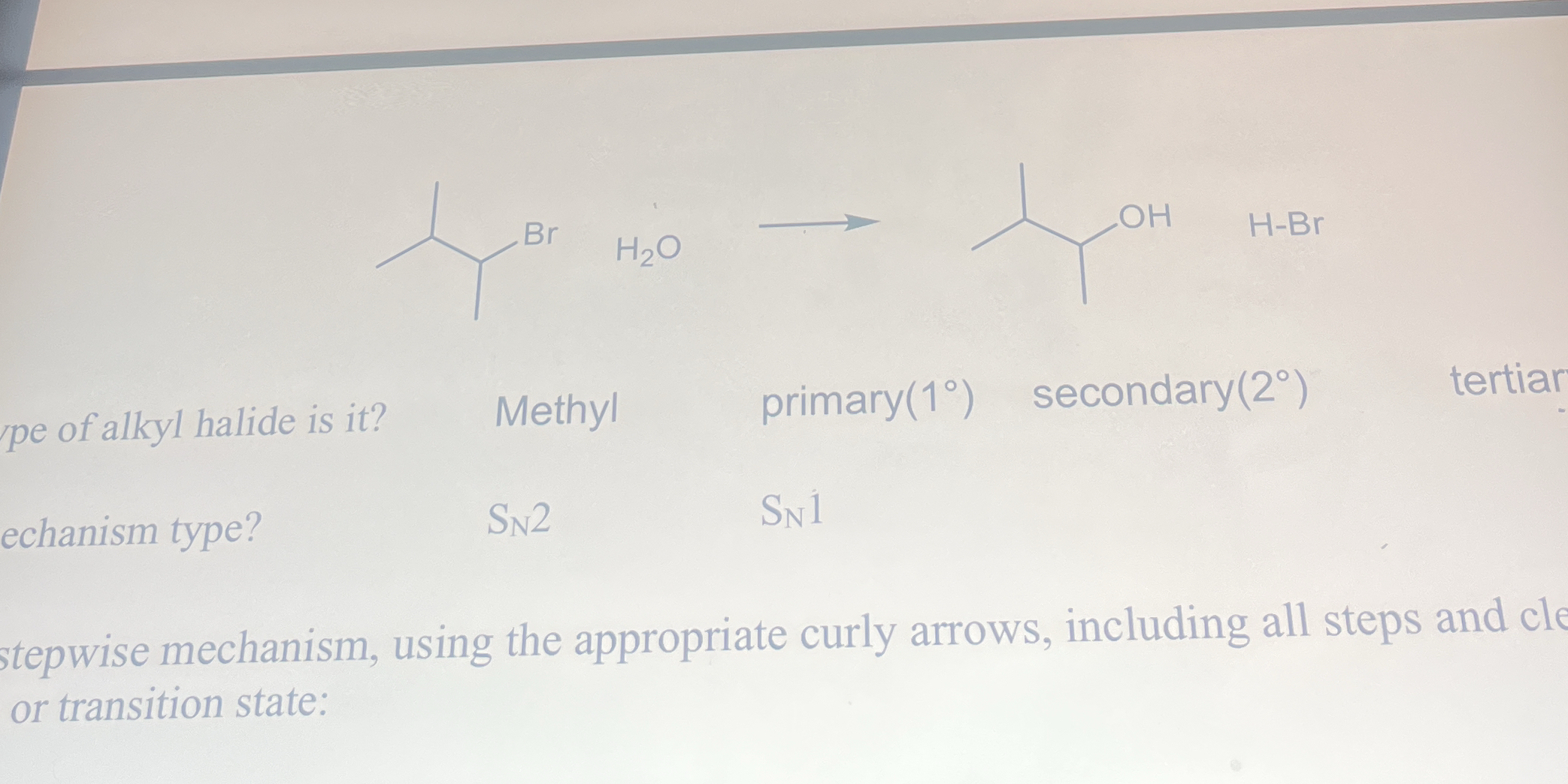
What type of alkyl halide is it? What is the mechanism type? Show the stepwise mechanism using the appropriate curly arrows, including all steps and clear transition state.
Understand the Problem
The question is asking for the identification of the type of alkyl halide and the corresponding reaction mechanism for a chemical reaction involving a bromide and water. Additionally, it requests a stepwise mechanism with curly arrows to illustrate the process clearly.
Answer
Secondary alkyl halide, SN1 mechanism.
The final answer is secondary alkyl halide and SN1 mechanism.
Answer for screen readers
The final answer is secondary alkyl halide and SN1 mechanism.
More Information
Secondary alkyl halides like the one shown typically undergo substitution reactions. Since water is a weak nucleophile, the reaction is more likely to proceed via an SN1 mechanism with the formation of a stable carbocation.
Tips
A common mistake is confusing SN1 with SN2 reactions. Remember, SN1 often occurs with weak nucleophiles and forms a carbocation intermediate.
Sources
- Substitution reactions of alkyl halides: two mechanisms - chem.libretexts.org
- The SN1 Reaction Mechanism - Master Organic Chemistry - masterorganicchemistry.com
AI-generated content may contain errors. Please verify critical information