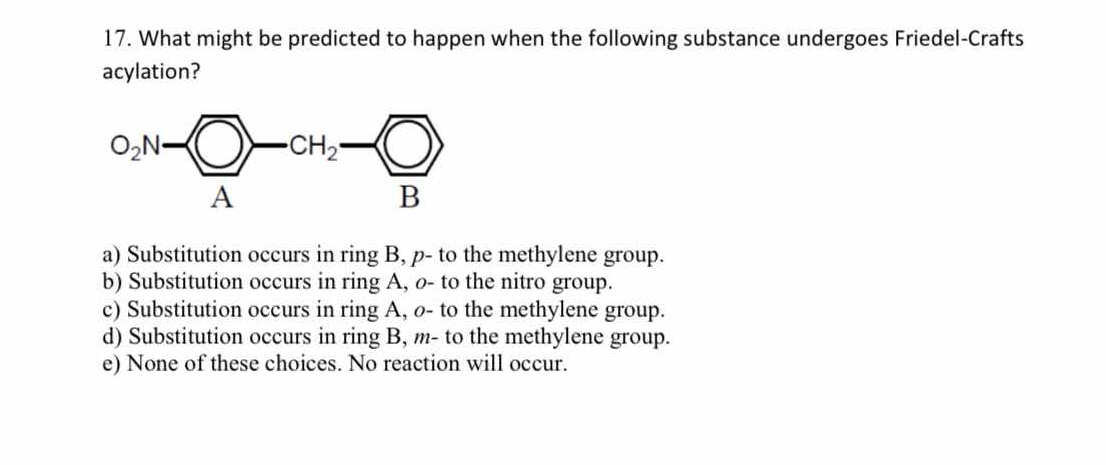
What might be predicted to happen when the following substance undergoes Friedel-Crafts acylation?
Understand the Problem
The question is asking about the expected outcomes when a specific chemical compound undergoes Friedel-Crafts acylation, focusing on the substitution patterns that may occur in two aromatic rings labeled A and B.
Answer
Substitution occurs in ring B, p- to the methylene group.
The final answer is substitution occurs in ring B, p- to the methylene group.
Answer for screen readers
The final answer is substitution occurs in ring B, p- to the methylene group.
More Information
In electrophilic aromatic substitution reactions like Friedel-Crafts acylation, the presence of electron-withdrawing groups like NO2 deactivates the ring for further substitution, while ring B remains more reactive.
Tips
A common mistake is not considering the deactivating effect of the nitro group, which prevents acylation in ring A.
Sources
AI-generated content may contain errors. Please verify critical information