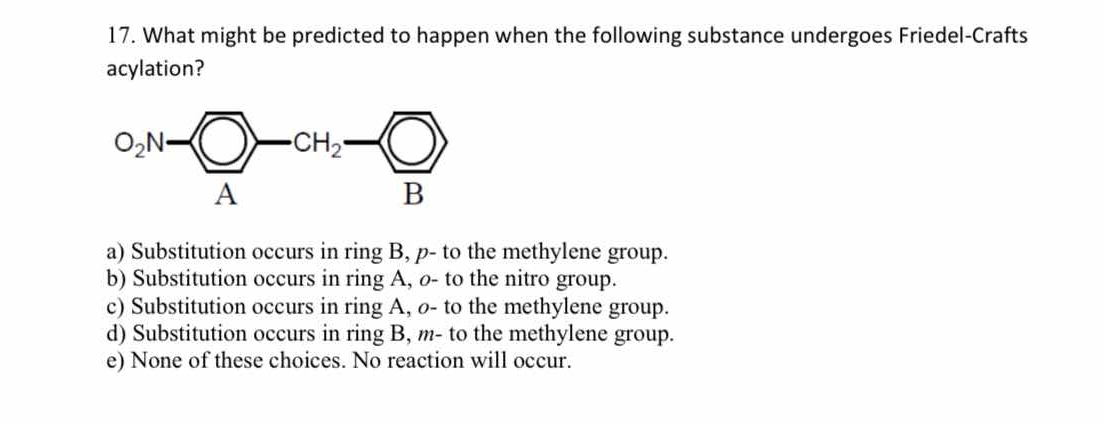
What might be predicted to happen when the following substance undergoes Friedel-Crafts acylation?
Understand the Problem
The question is asking about the predicted outcome of a Friedel-Crafts acylation reaction involving a specific compound. It requires understanding of electrophilic aromatic substitution and the effects of substituents on the aromatic rings.
Answer
Substitution in ring B, p- to the methylene group.
The final answer is substitution occurs in ring B, p- to the methylene group.
Answer for screen readers
The final answer is substitution occurs in ring B, p- to the methylene group.
More Information
The nitro group on ring A is a strong deactivating group that directs substitution to the meta position. However, its presence deactivates the ring, making ring B, which is more distant from the nitro group, more favorable for reaction. Thus, substitution is expected to occur para to the methylene bridge on ring B.
Tips
A common mistake is forgetting that strong deactivators like nitro groups make certain rings less reactive, shifting substitution to a position on a different, less hindered ring.
Sources
AI-generated content may contain errors. Please verify critical information