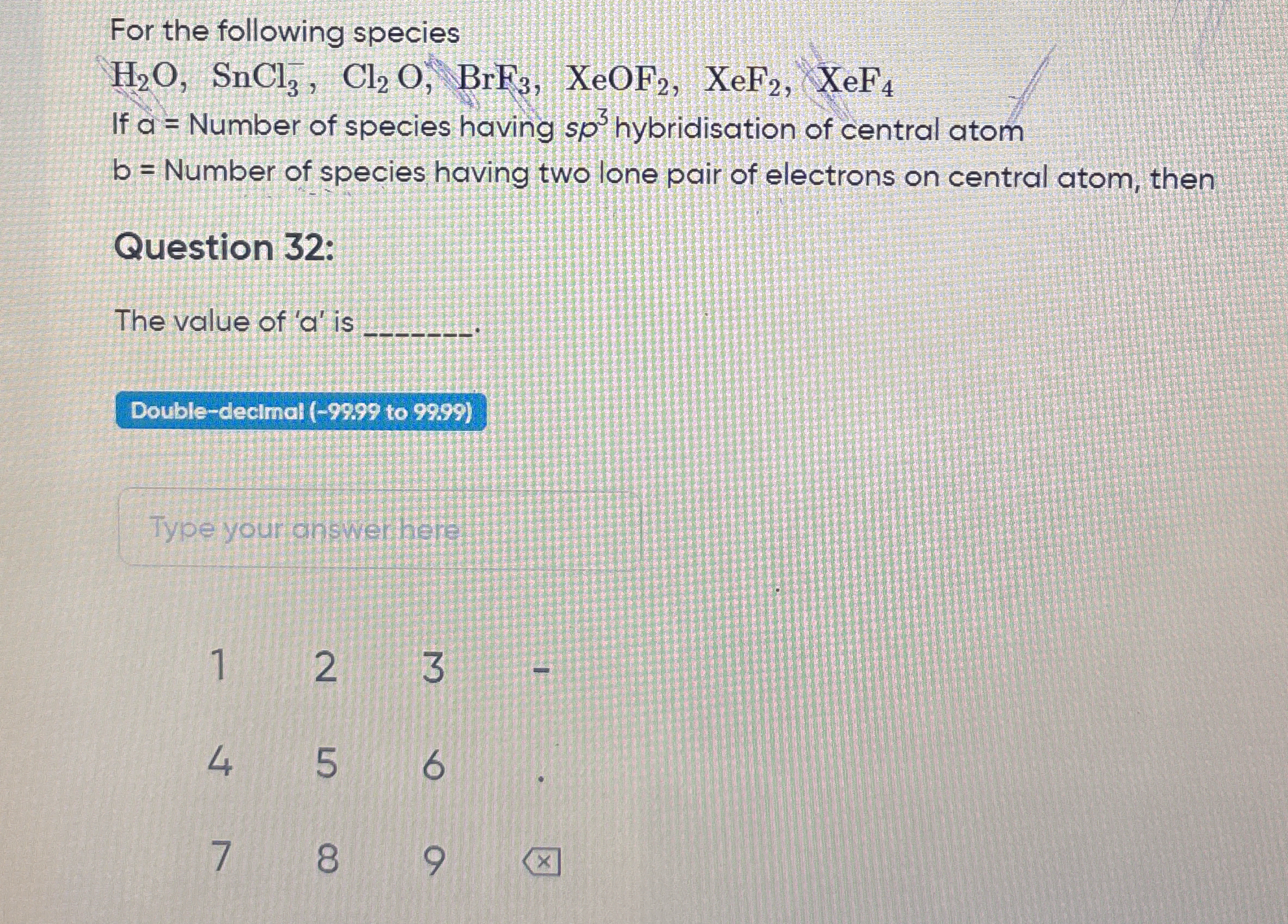
What is the value of 'a' for the given species, which is the number of species having sp³ hybridization of the central atom?
Understand the Problem
The question asks for the calculation of the variable 'a', which represents the number of species from a given list that have sp³ hybridization of the central atom. The list includes several chemical species, and the user needs to analyze their hybridization to determine the right answer.
Answer
2
The final answer is 2.
Answer for screen readers
The final answer is 2.
More Information
Species like H2O and Cl2O have an sp³ hybridized central atom. These compounds use one s-orbital and three p-orbitals to form four equivalent sp³ orbitals.
Tips
A common mistake is to miscalculate the hybridization by not considering lone pairs in the structure.
Sources
- 5.2D: sp3 Hybridization - Chemistry LibreTexts - chem.libretexts.org
- How To Determine Hybridization: A Shortcut - masterorganicchemistry.com
AI-generated content may contain errors. Please verify critical information