What is the significance of hydrogen bonds in water, and how do they affect its physical properties like melting point, boiling point, and heat of vaporization?
Understand the Problem
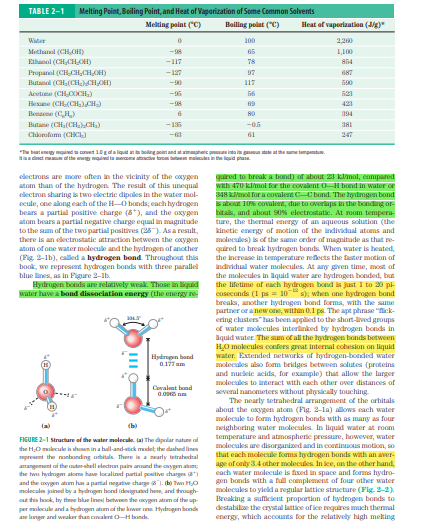
The question is referencing a table and figure related to the properties of common solvents, particularly focusing on aspects like melting point, boiling point, and heat of vaporization. It seems to be exploring the concept of hydrogen bonding and its effects on water's properties.
Answer
Hydrogen bonds cause water's high melting/boiling points and heat of vaporization.
The hydrogen bonds in water lead to high melting and boiling points, high heat of vaporization, and strong cohesion. These bonds require significant energy to break, stabilizing water's liquid state and playing a critical role in regulating Earth's climate and biological functions.
Answer for screen readers
The hydrogen bonds in water lead to high melting and boiling points, high heat of vaporization, and strong cohesion. These bonds require significant energy to break, stabilizing water's liquid state and playing a critical role in regulating Earth's climate and biological functions.
More Information
Water's unique properties, driven by hydrogen bonding, are crucial for life's processes and Earth's climate regulation. These bonds make water an effective heat regulator and solvent, supporting diverse ecosystems.
Tips
A common mistake is to underestimate the strength and impact of hydrogen bonds compared to other intermolecular forces. Ensure to highlight their role in water's high thermal properties.
Sources
- Hydrogen bonds in water (article) - Khan Academy - khanacademy.org
- Unusual Properties of Water - Chemistry LibreTexts - chem.libretexts.org
- 5.1 Properties of Water – Introduction to Oceanography - rwu.pressbooks.pub
AI-generated content may contain errors. Please verify critical information