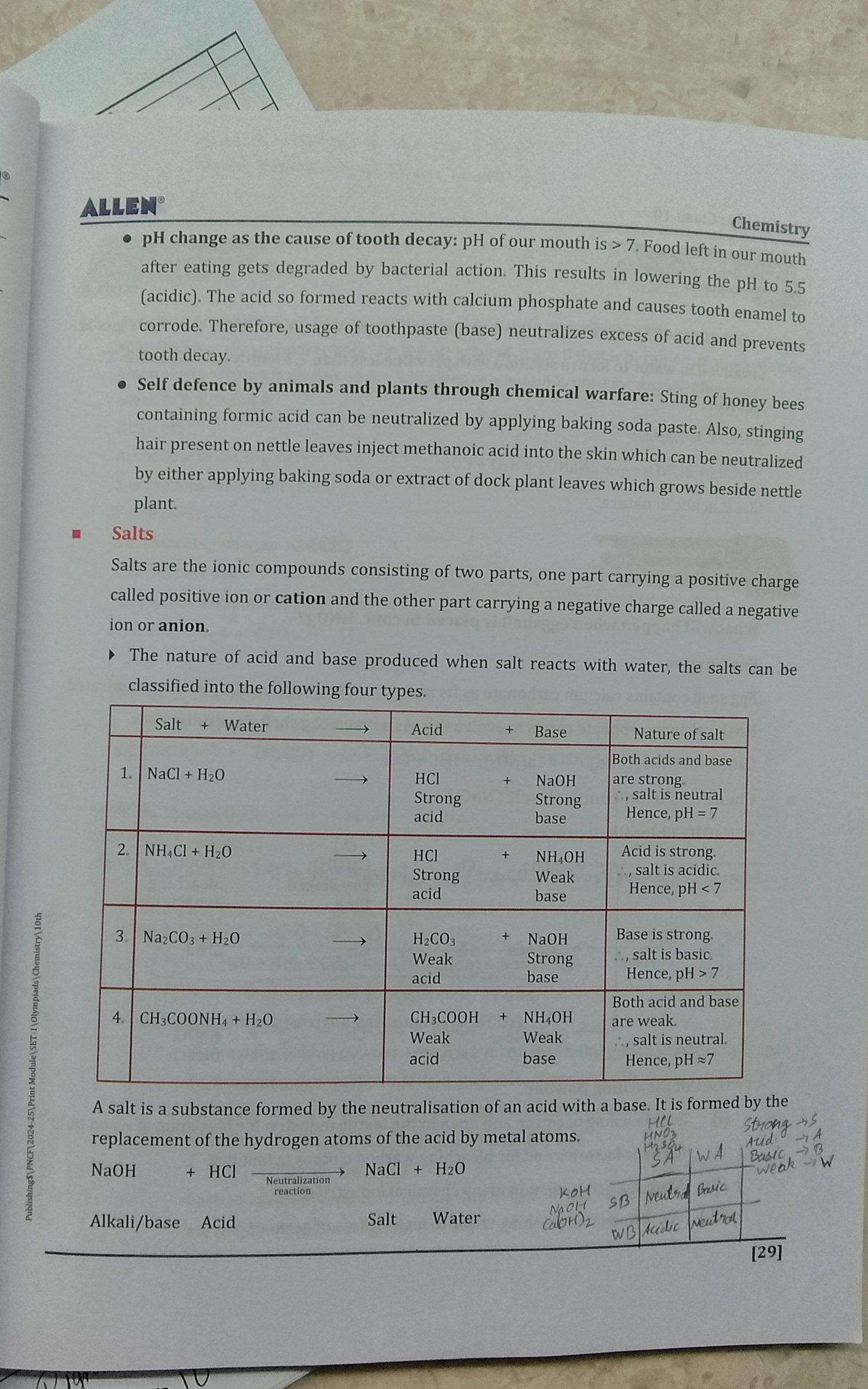
What is the relationship between pH change and tooth decay, and how do salts react with water?
Understand the Problem
The question is summarizing concepts related to pH changes, tooth decay, chemical defenses in animals, and reactions between salts and water. It's intended to provide information about how pH impacts dental health and the chemical reactions involving salts, acids, and bases.
Answer
Tooth decay starts below pH 5.5; salts affect water pH based on constituent acid/base strength.
Tooth decay occurs when mouth pH drops below 5.5, leading to enamel demineralization. Salts react with water to form acidic, basic, or neutral solutions depending on the strengths of the acid and base components.
Answer for screen readers
Tooth decay occurs when mouth pH drops below 5.5, leading to enamel demineralization. Salts react with water to form acidic, basic, or neutral solutions depending on the strengths of the acid and base components.
More Information
Tooth decay is mainly a result of bacterial acids in food debris causing a drop in pH, which damages the tooth structure.
Tips
Misunderstanding can occur if the strengths of the reacting acid and base are not considered when predicting pH changes in salts.
Sources
- Explain the pH Change As the Cause Of Tooth Decay - geeksforgeeks.org
- How Salts Affect Water pH - .chem.wisc.edu
AI-generated content may contain errors. Please verify critical information