What is the relationship between ΔU° of combustion of methane and ΔH°? The enthalpy of combustion of methane is -X kJ mol-1. What will be the enthalpy of formation of CH4(g)?
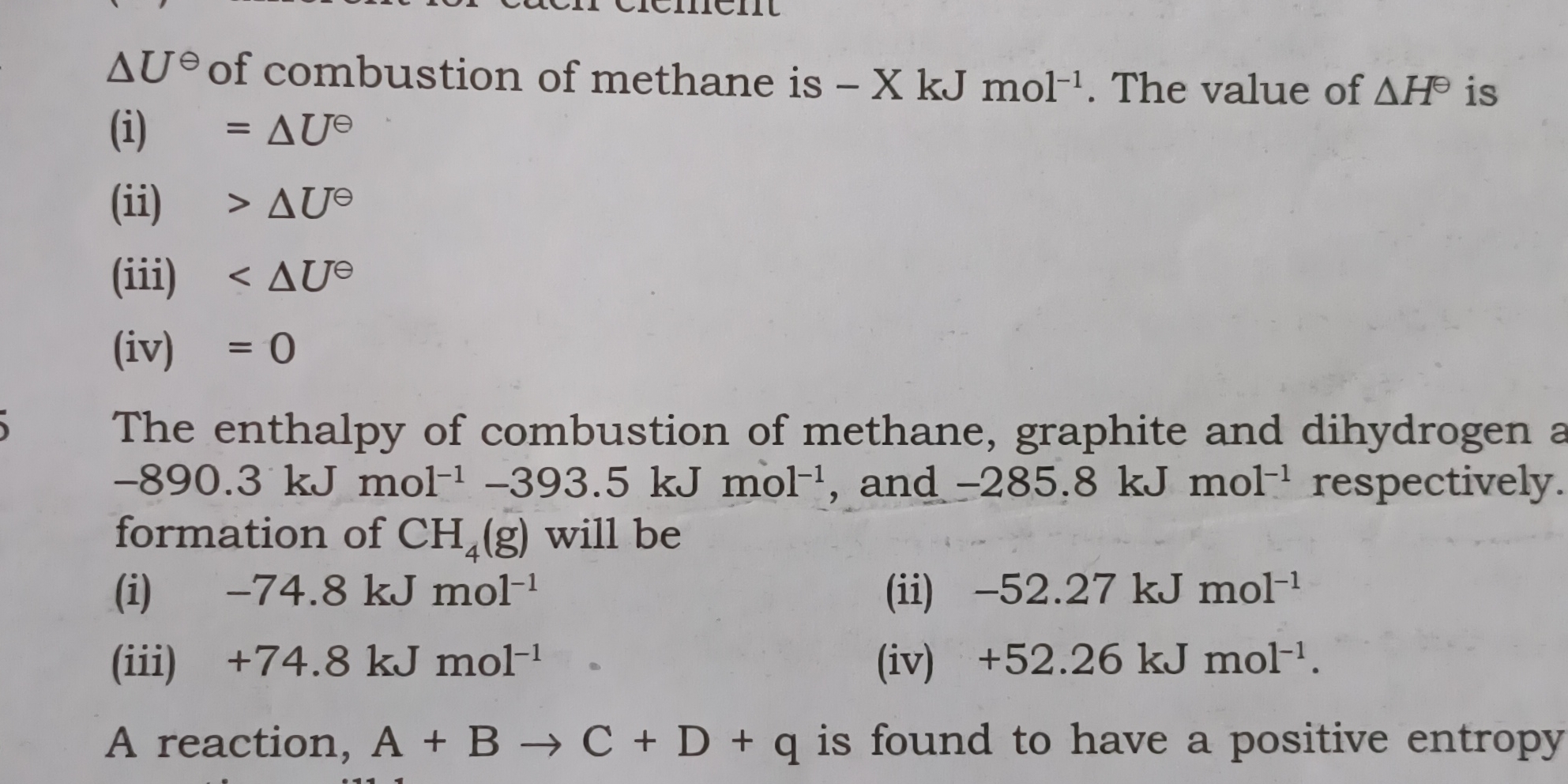
Understand the Problem
The question appears to involve thermodynamics, specifically enthalpy and internal energy changes associated with the combustion of methane. It asks for a comparison of ΔU° (internal energy change) with respect to ΔH° (enthalpy change) and the enthalpy of formation of CH4, following a given reaction. The question seeks to understand the relationships among these thermodynamic quantities.
Answer
-74.8 kJ/mol
The enthalpy of formation of CH4(g) is -74.8 kJ/mol.
Answer for screen readers
The enthalpy of formation of CH4(g) is -74.8 kJ/mol.
More Information
The enthalpy of formation represents the energy change when one mole of a compound is formed from its elements in their standard states.
Tips
Remember to account for the change in moles of gas (Δn) in calculations involving gases and standard conditions.
Sources
AI-generated content may contain errors. Please verify critical information