What is the reaction of acyl chlorides with ammonia and amines to form amides?
Understand the Problem
The question is likely related to the chemical reactions of acyl chlorides with ammonia and amines to form amides. It involves understanding the mechanisms and products of these reactions.
Answer
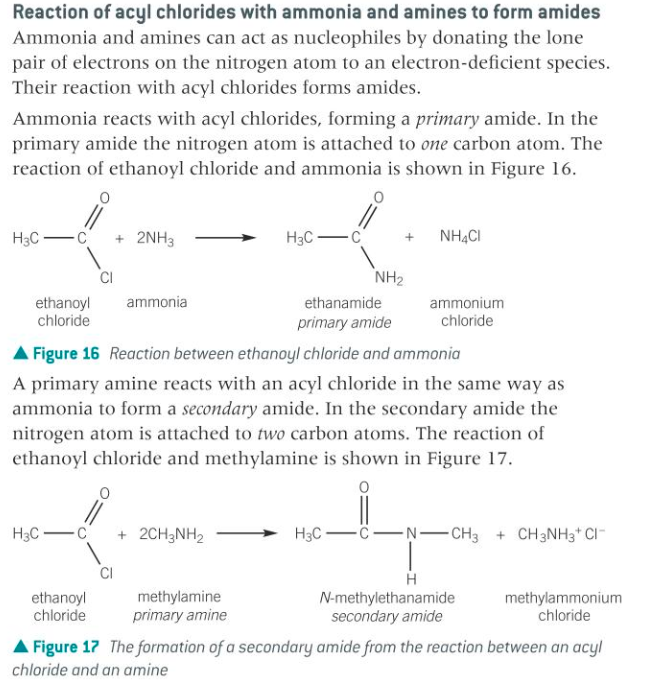
Acyl chlorides react with ammonia to form primary amides, and with primary and secondary amines to form secondary and tertiary amides respectively.
Acyl chlorides react with ammonia and amines to form primary, secondary, and tertiary amides respectively. In these reactions, ammonia or amines act as nucleophiles, attacking the electron-deficient carbonyl carbon of the acyl chloride, resulting in the substitution of the chloride group with an amide group.
Answer for screen readers
Acyl chlorides react with ammonia and amines to form primary, secondary, and tertiary amides respectively. In these reactions, ammonia or amines act as nucleophiles, attacking the electron-deficient carbonyl carbon of the acyl chloride, resulting in the substitution of the chloride group with an amide group.
More Information
The reaction between acyl chlorides and ammonia/amines is a nucleophilic acyl substitution, yielding various amides. This type of reaction is known as aminolysis.
Tips
Ensure to distinguish between the types of amines (primary, secondary, tertiary) as they form different amide products. Also, recognize the nucleophilic nature of ammonia and amines in the context of this reaction.
Sources
- Acid chlorides react with ammonia, 1° amines and 2° amines to form amides - chem.libretexts.org
- acyl chlorides with ammonia or primary amines - Chemguide - chemguide.co.uk
- Making Amides from Acyl Chlorides - Chemistry LibreTexts - chem.libretexts.org
AI-generated content may contain errors. Please verify critical information