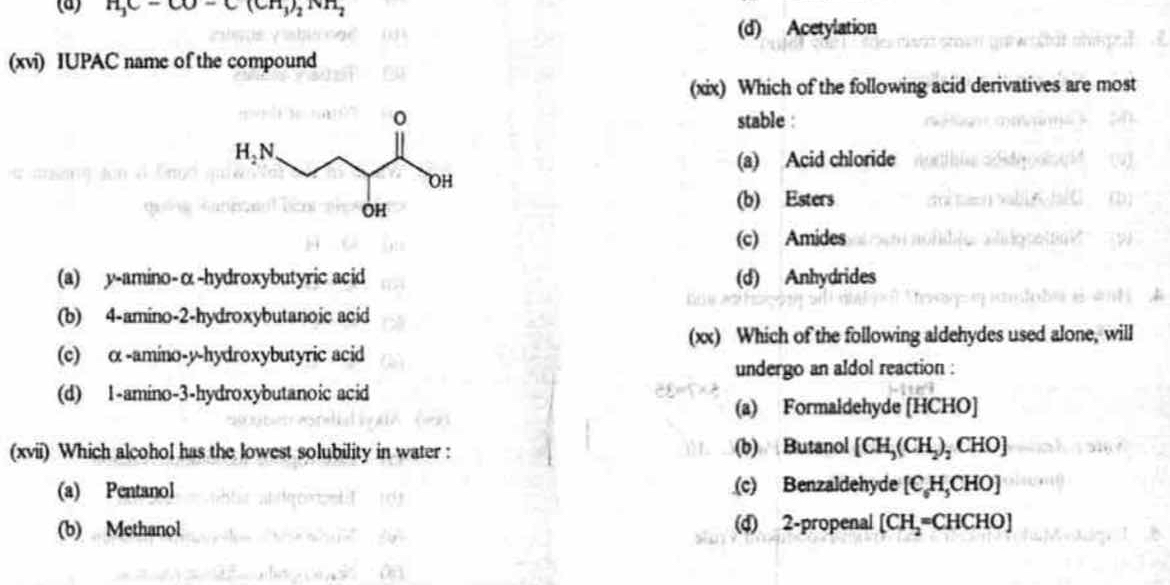
What is the IUPAC name of the compound? Which alcohol has the lowest solubility in water? Which of the following acid derivatives are most stable? Which aldehydes will undergo an a... What is the IUPAC name of the compound? Which alcohol has the lowest solubility in water? Which of the following acid derivatives are most stable? Which aldehydes will undergo an aldol reaction?
Understand the Problem
The question involves chemical nomenclature and reactions, specifically asking for the IUPAC name of a compound, stability of acid derivatives, and conditions for aldol reactions.
Answer
4-amino-2-hydroxybutanoic acid; Pentanol; Amides; Butanal
The IUPAC name of the compound is 4-amino-2-hydroxybutanoic acid. Pentanol has the lowest solubility in water. Amides are the most stable acid derivatives. Butanal will undergo an aldol reaction.
Answer for screen readers
The IUPAC name of the compound is 4-amino-2-hydroxybutanoic acid. Pentanol has the lowest solubility in water. Amides are the most stable acid derivatives. Butanal will undergo an aldol reaction.
More Information
4-amino-2-hydroxybutanoic acid correctly represents the structure shown. Pentanol, being a longer chain alcohol, is less soluble in water than methanol. Amides are more stable due to resonance. Butanal, with an alpha hydrogen, can undergo an aldol reaction.
Tips
Focus on identifying functional groups correctly for IUPAC naming and considering the effects of molecular structure on solubility and stability.
Sources
- Carboxyl Derivatives - Chemistry LibreTexts - chem.libretexts.org
- Acetaldehyde - Wikipedia - en.wikipedia.org
- Alcohols - Nomenclature and Properties - Master Organic Chemistry - masterorganicchemistry.com
AI-generated content may contain errors. Please verify critical information