What is osmotic pressure and how does water move through aquaporins?
Understand the Problem
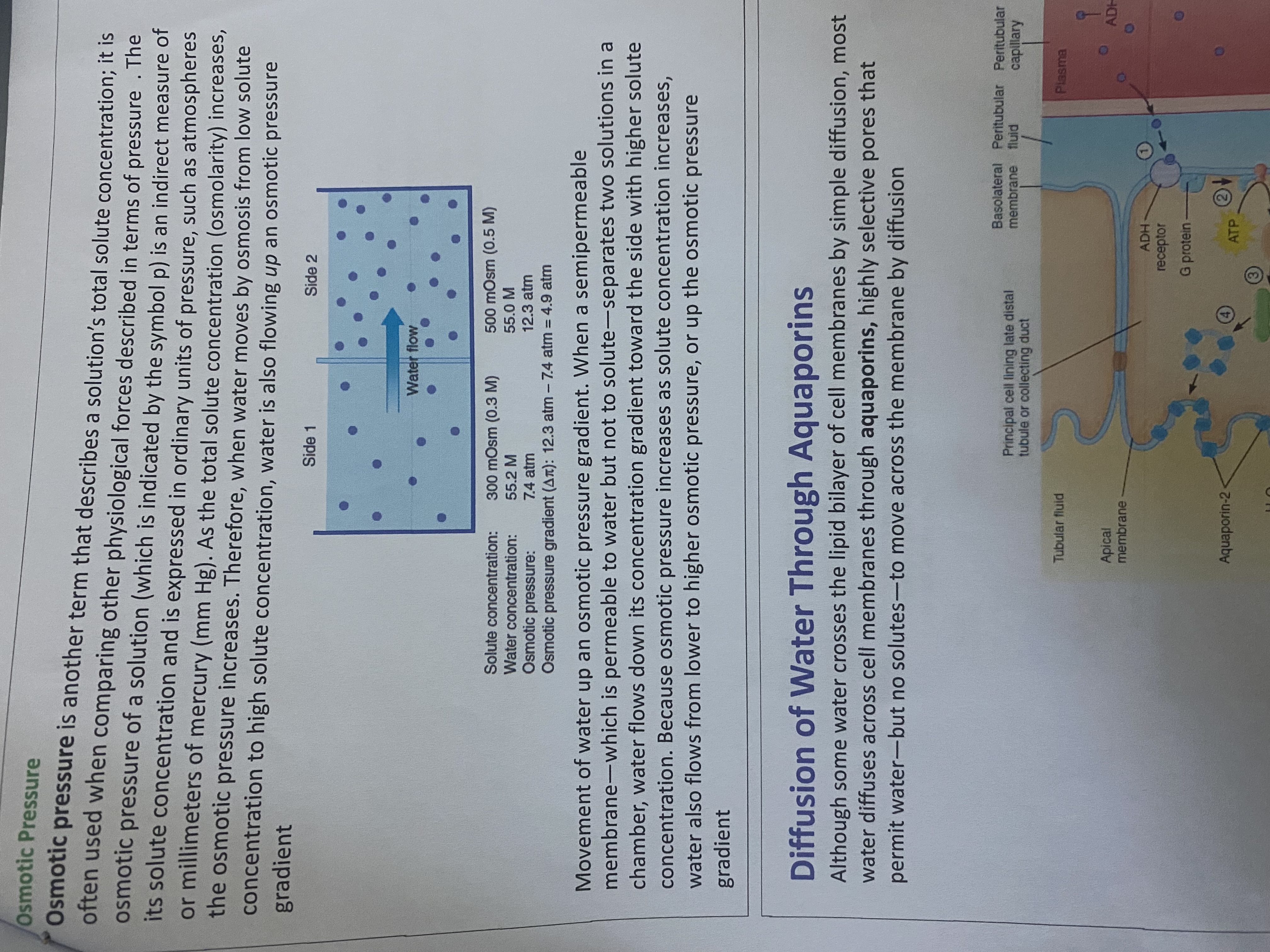
The question discusses the concept of osmotic pressure and how water movement is affected by concentration gradients. It may also explore the role of aquaporins in water diffusion across cell membranes.
Answer
Osmotic pressure drives water through aquaporins from low to high solute concentration areas.
Osmotic pressure is the pressure required to stop water from diffusing through a membrane via osmosis. Water moves through aquaporins, proteins that form channels in cell membranes, allowing water to move from areas of low to high solute concentration, following osmotic pressure gradients.
Answer for screen readers
Osmotic pressure is the pressure required to stop water from diffusing through a membrane via osmosis. Water moves through aquaporins, proteins that form channels in cell membranes, allowing water to move from areas of low to high solute concentration, following osmotic pressure gradients.
More Information
Osmotic pressure plays a crucial role in maintaining cell turgor and fluid balance in organisms. Aquaporins enable rapid and regulated water movement across cell membranes.
Tips
A common mistake is to confuse osmotic pressure with simple diffusion. Osmotic pressure deals specifically with water movement across a semipermeable membrane.
Sources
- Osmosis, Tonicity, and Hydrostatic Pressure - vivo.colostate.edu
- Aquaporins: Highly Regulated Channels Controlling Plant Water ... - ncbi.nlm.nih.gov
AI-generated content may contain errors. Please verify critical information