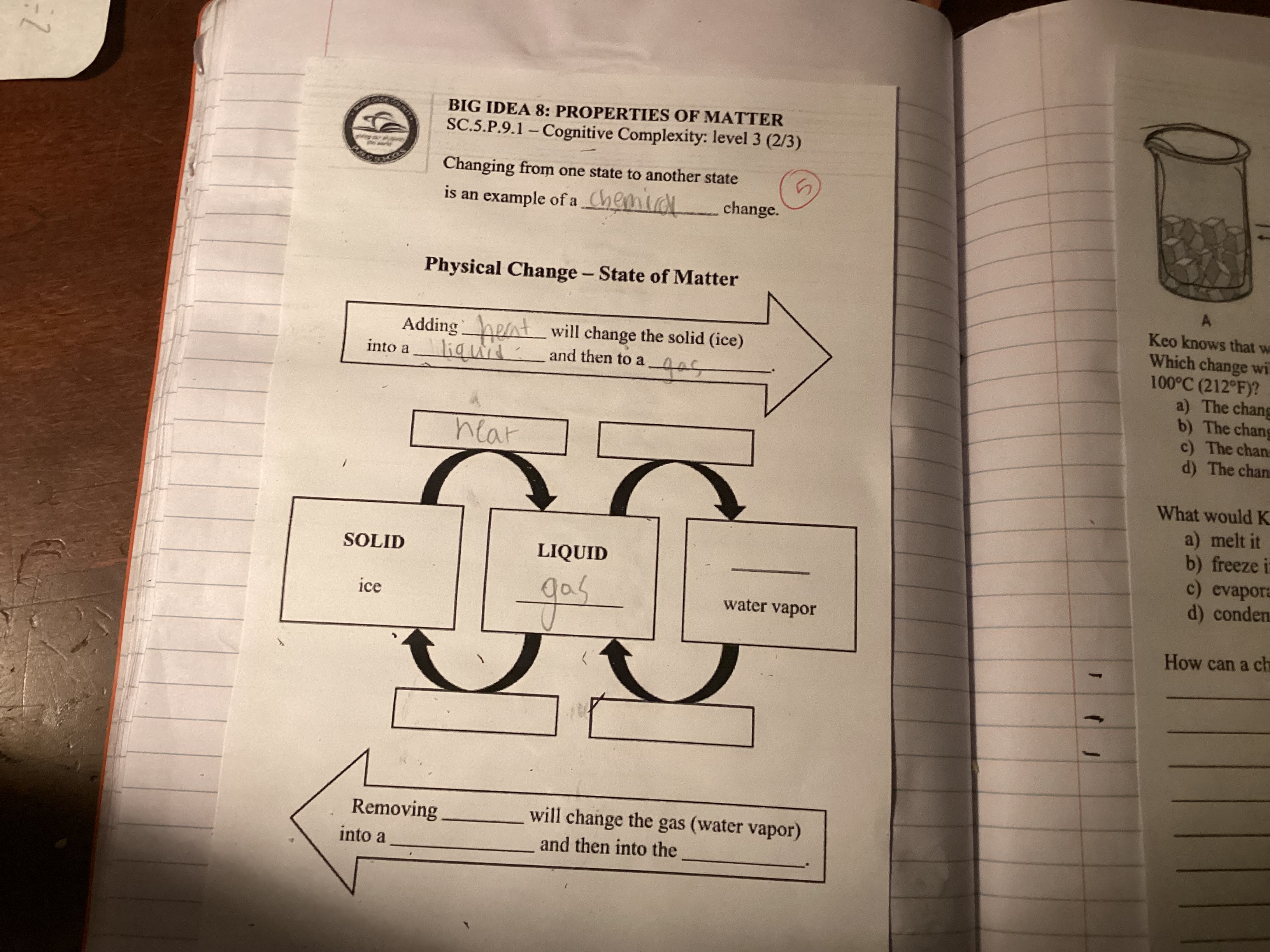
What changes occur when heat is added to a solid and what happens when heat is removed from a gas?
Understand the Problem
The question is asking about the processes of changing states of matter and what happens when heat is added or removed. It requires identifying the changes associated with these processes and provides a diagram related to physical changes in matter.
Answer
Add heat to a solid: solid to liquid to gas. Remove heat from gas: gas to liquid to solid.
Adding heat to a solid causes it to melt into a liquid and potentially become a gas if enough heat is added. Removing heat from a gas causes it to condense into a liquid and eventually freeze into a solid.
Answer for screen readers
Adding heat to a solid causes it to melt into a liquid and potentially become a gas if enough heat is added. Removing heat from a gas causes it to condense into a liquid and eventually freeze into a solid.
More Information
These processes are examples of phase changes due to the transfer of thermal energy, affecting particle movement and distance.
Tips
A common mistake is assuming substances skip phases, like gas directly to solid, under normal conditions.
Sources
- Modeling Changes in State of Matter - study.com
- Phase Changes - Chemistry LibreTexts - chem.libretexts.org
- Phase Changes – Chemistry of Food and Cooking - mhcc.pressbooks.pub
AI-generated content may contain errors. Please verify critical information