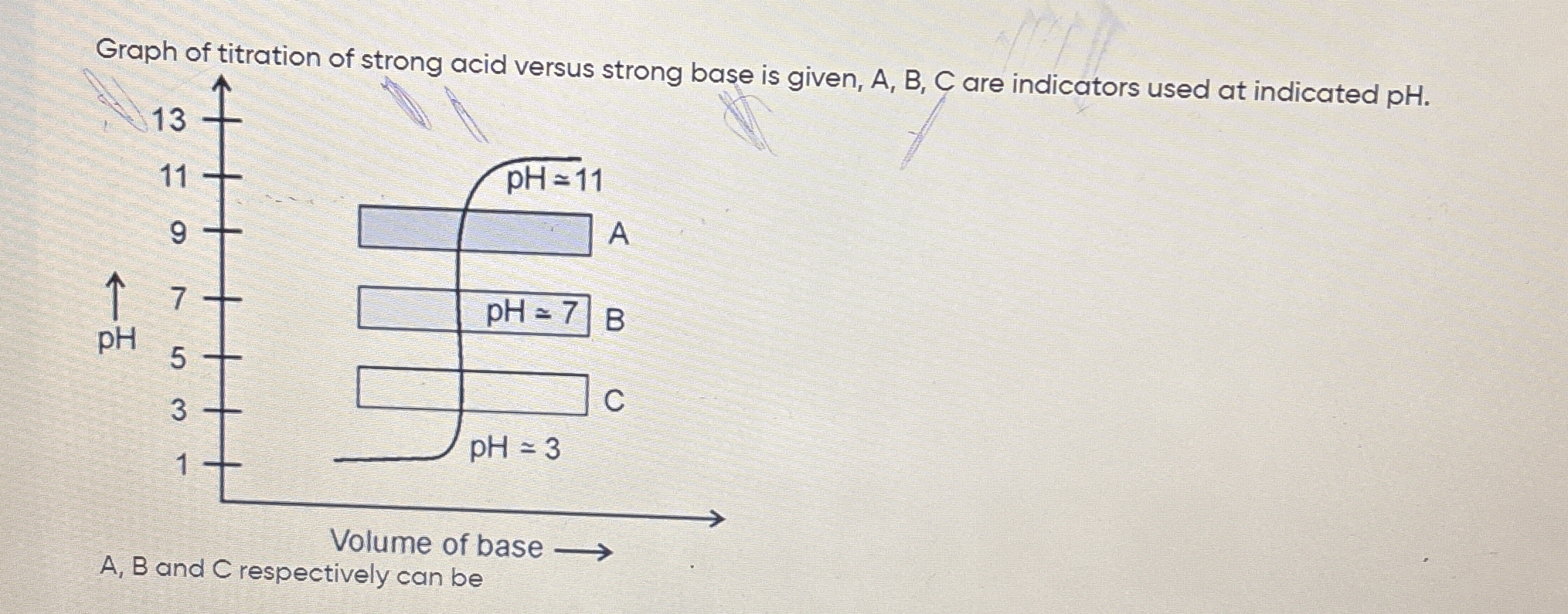
What are the roles of indicators A, B, and C at the indicated pH levels in the titration graph of a strong acid versus a strong base?
Understand the Problem
The question is asking about the interpretation of a titration graph, specifically identifying the roles of indicators A, B, and C at the indicated pH levels during a titration of a strong acid with a strong base.
Answer
A: Phenolphthalein, B: Bromothymol Blue, C: Methyl Orange
A: Phenolphthalein (pH 11), B: Bromothymol Blue (pH 7), C: Methyl Orange (pH 3)
Answer for screen readers
A: Phenolphthalein (pH 11), B: Bromothymol Blue (pH 7), C: Methyl Orange (pH 3)
More Information
Phenolphthalein changes color around pH 8-10, making it suitable for basic conditions. Bromothymol Blue is effective around pH 6-7, appropriate for neutral conditions. Methyl Orange changes color in slightly acidic conditions, around pH 3.1-4.4.
Tips
Using an indicator whose pKa aligns with the pH at the equivalence point of the titration provides the most accurate results.
Sources
- Choosing Acid-Base Titration Indicators Chemistry Tutorial - ausetute.com.au
- Acid-Base Titrations | Chemistry for Majors - Lumen - courses.lumenlearning.com
AI-generated content may contain errors. Please verify critical information