What are the reactions of acyl chlorides with ammonia and amines to form amides?
Understand the Problem
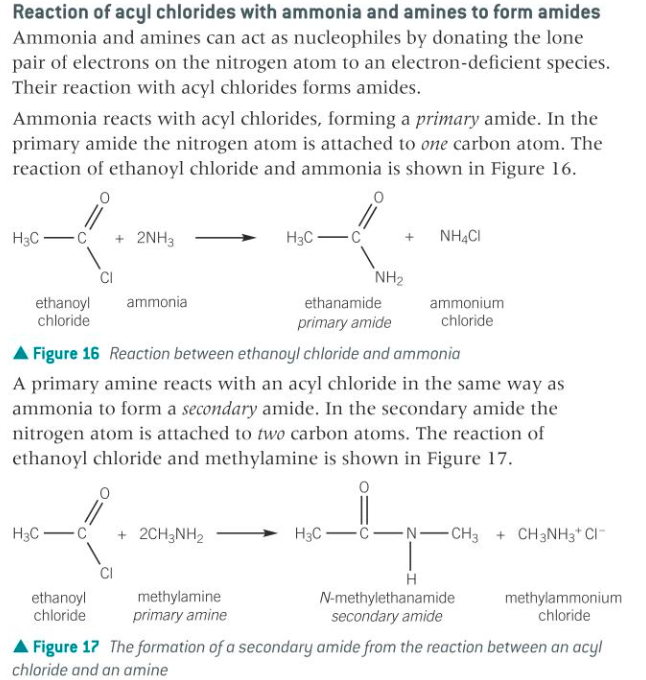
The question is about the reaction of acyl chlorides with ammonia and amines to form amides. It discusses how ammonia leads to the formation of primary amides and how primary amines react to form secondary amides, detailing the respective reactions and compounds involved.
Answer
Acyl chlorides react with ammonia to form primary amides and with primary amines to form secondary amides.
The reactions of acyl chlorides with ammonia and primary amines form primary and secondary amides respectively, releasing byproducts like hydrogen chloride and ammonium salts.
Answer for screen readers
The reactions of acyl chlorides with ammonia and primary amines form primary and secondary amides respectively, releasing byproducts like hydrogen chloride and ammonium salts.
More Information
Acyl chlorides are highly reactive compounds that are commonly used in organic syntheses to form amides. The nucleophilic attack by the nitrogen on the carbonyl carbon is key to these reactions.
Tips
A common mistake is to neglect the formation of byproducts such as hydrogen chloride or ammonium salts. Always balance the equations to include all products.
Sources
- Making Amides from Acyl Chlorides - chem.libretexts.org
- Acid Chlorides react with Ammonia, 1° Amines, and 2° Amines - chem.libretexts.org
- Reaction between Acyl Chlorides and Ammonia - chemguide.co.uk
AI-generated content may contain errors. Please verify critical information