What are the principles and requirements for electrolysis?
Understand the Problem
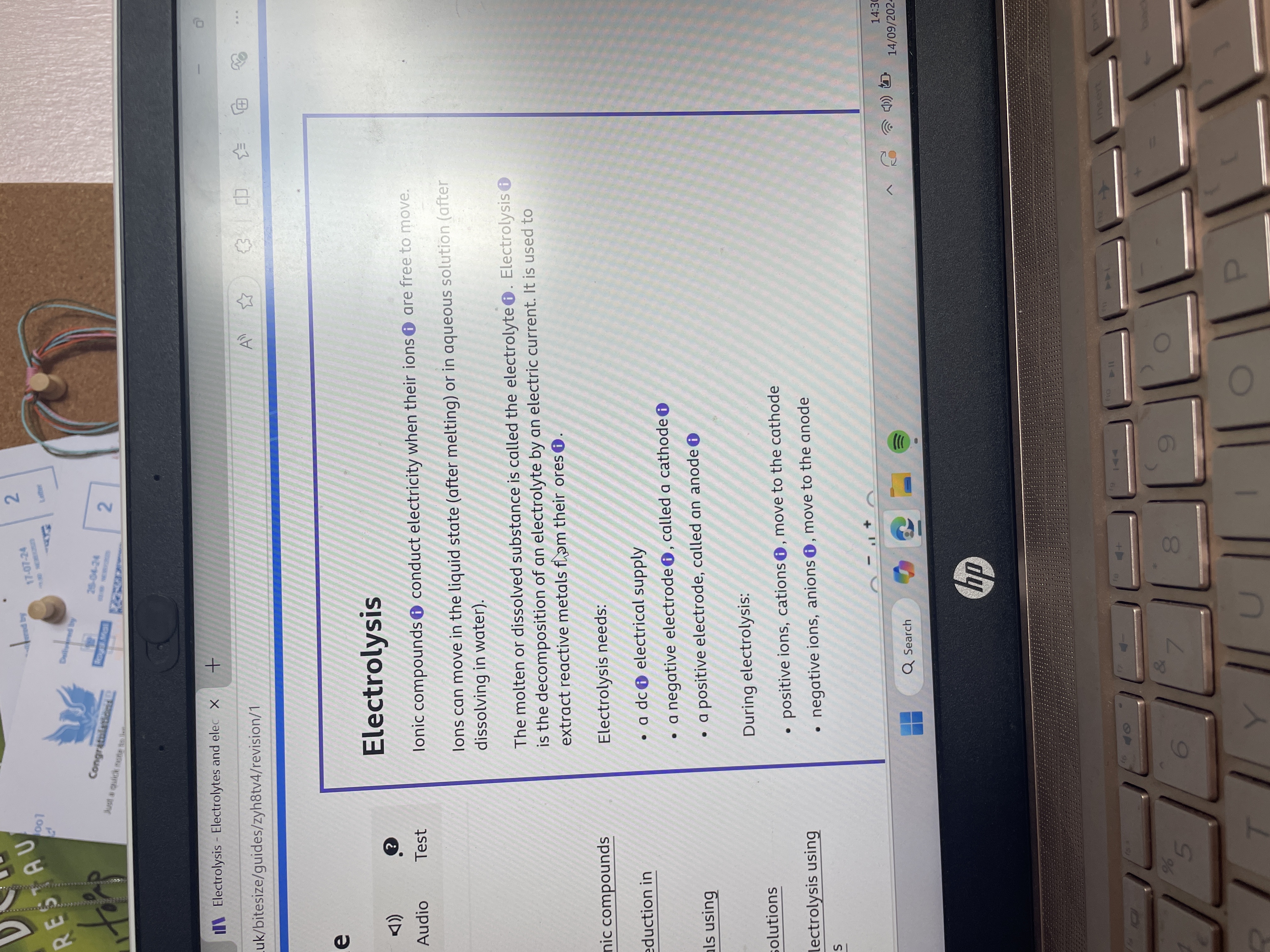
The question appears to be related to the topic of electrolysis, specifically focusing on the principles of how ionic compounds conduct electricity and the requirements for the process of electrolysis.
Answer
Electrolysis requires a dc supply, an electrolyte, a cathode, and an anode. Cations move to the cathode, and anions move to the anode. The electrolyte must be molten or in an aqueous state.
The principles and requirements for electrolysis involve using a dc electrical supply, an electrolyte, a cathode, and an anode. During electrolysis, cations move to the cathode, and anions move to the anode. The process requires ions to be in a molten or aqueous state for mobility.
Answer for screen readers
The principles and requirements for electrolysis involve using a dc electrical supply, an electrolyte, a cathode, and an anode. During electrolysis, cations move to the cathode, and anions move to the anode. The process requires ions to be in a molten or aqueous state for mobility.
More Information
During electrolysis, the anode undergoes oxidation, and the cathode undergoes reduction. This process is often used in industries for the extraction of metals and production of chemicals.
Tips
A common mistake in electrolysis is using an incorrect voltage or electrolyte concentration, which can disrupt the process and lead to undesired reactions. Always ensure the correct setup and conditions.
Sources
- Principles of Electrolysis - Chemist Ai - chemistai.org
- Principle of Water Electrolysis, Important Factors, Electrolytes - BYJU'S - byjus.com
- Introduction to the principles of electroplating and electrolysis - timonic.com.cn