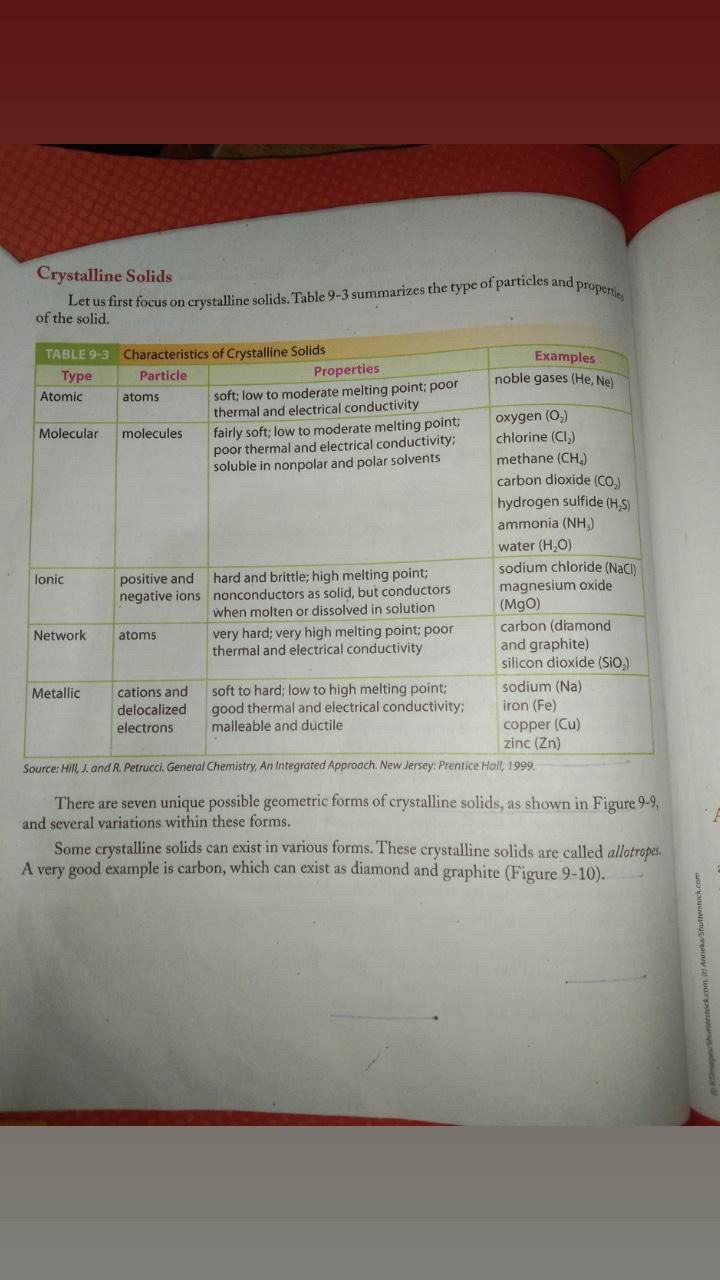
What are the characteristics and examples of different types of crystalline solids?
Understand the Problem
The question is asking about the characteristics of different types of crystalline solids, as outlined in the provided table. The inquiry pertains to the properties and examples of various crystalline solid types, such as atomic, molecular, ionic, network, and metallic solids.
Answer
Crystalline solids: atomic, molecular, ionic, network, and metallic types.
Crystalline solids are categorized into atomic, molecular, ionic, network, and metallic types, each with distinct particles and properties.
Answer for screen readers
Crystalline solids are categorized into atomic, molecular, ionic, network, and metallic types, each with distinct particles and properties.
More Information
Each type of crystalline solid has unique properties. Atomic solids have atoms bonded together; molecular solids consist of molecules; ionic solids are formed from electrostatic attractions; covalent network solids have a network of bonds; metallic solids involve a sea of electrons.
Tips
A common mistake is confusing the properties and examples of each type. Ensure clear understanding of the unique particle and bonding characteristics of each solid type.
Sources
- 12.7: Types of Crystalline Solids- Molecular, Ionic, and Atomic - chem.libretexts.org
- Classes of Crystalline Solids - CK12-Foundation - flexbooks.ck12.org
AI-generated content may contain errors. Please verify critical information