What are the changes in the states of matter?
Understand the Problem
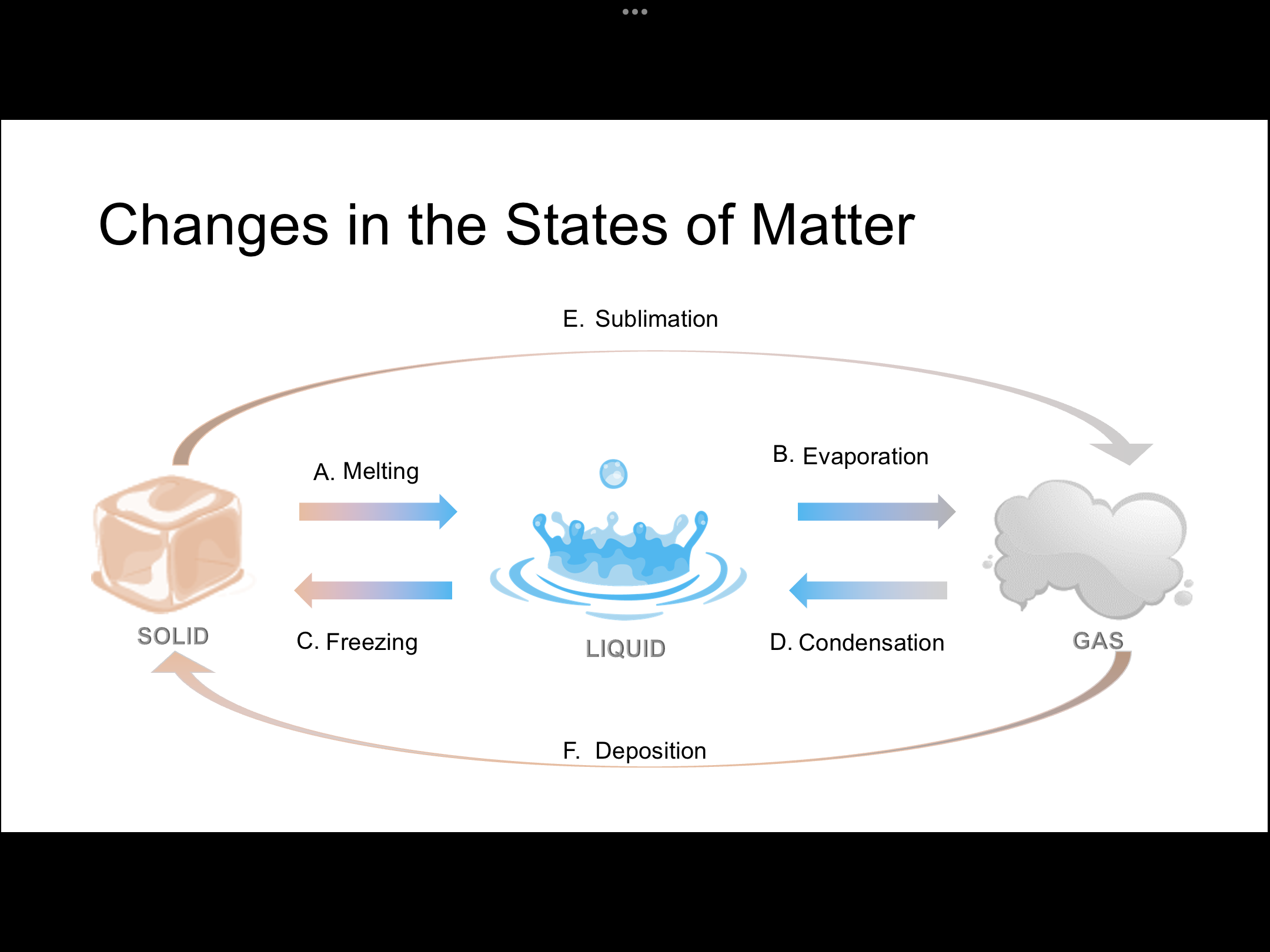
The image illustrates the various changes in the states of matter, showing the transitions between solid, liquid, and gas. It highlights processes like melting, freezing, evaporation, condensation, sublimation, and deposition, allowing an understanding of how matter transitions between different states.
Answer
Melting, Evaporation, Freezing, Condensation, Sublimation, Deposition
The changes in the states of matter shown in the image are: A. Melting, B. Evaporation, C. Freezing, D. Condensation, E. Sublimation, F. Deposition.
Answer for screen readers
The changes in the states of matter shown in the image are: A. Melting, B. Evaporation, C. Freezing, D. Condensation, E. Sublimation, F. Deposition.
More Information
Matter changes state through various processes. Melting transforms a solid to a liquid, evaporation changes a liquid to a gas, freezing turns a liquid into a solid, condensation converts a gas into a liquid, sublimation changes a solid directly into a gas, and deposition transforms a gas directly into a solid.
Tips
A common mistake is to confuse sublimation with evaporation. Sublimation is the direct transition from solid to gas without passing through the liquid state, while evaporation is the change from liquid to gas.
Sources
AI-generated content may contain errors. Please verify critical information