What are hydrocarbons? Write structural formulae of the following compounds: propane, ethyne, cyclobutane, ethene, benzene.
Understand the Problem
The question is asking what hydrocarbons are and their classification, along with structural formula representation for specific compounds such as propane, ethyne, cyclobutane, ethene, and benzene.
Answer
Propane: H3C-CH2-CH3, Ethyne: HC≡CH, Cyclobutane: C4H8 (square ring), Ethene: H2C=CH2, Benzene: C6H6 (hexagonal ring).
Hydrocarbons are compounds composed only of carbon and hydrogen. The structural formulae are: Propane (H3C-CH2-CH3), Ethyne (HC≡CH), Cyclobutane (C4H8, in a square ring), Ethene (H2C=CH2), Benzene (C6H6, in a hexagonal ring with alternating double bonds).
Answer for screen readers
Hydrocarbons are compounds composed only of carbon and hydrogen. The structural formulae are: Propane (H3C-CH2-CH3), Ethyne (HC≡CH), Cyclobutane (C4H8, in a square ring), Ethene (H2C=CH2), Benzene (C6H6, in a hexagonal ring with alternating double bonds).
More Information
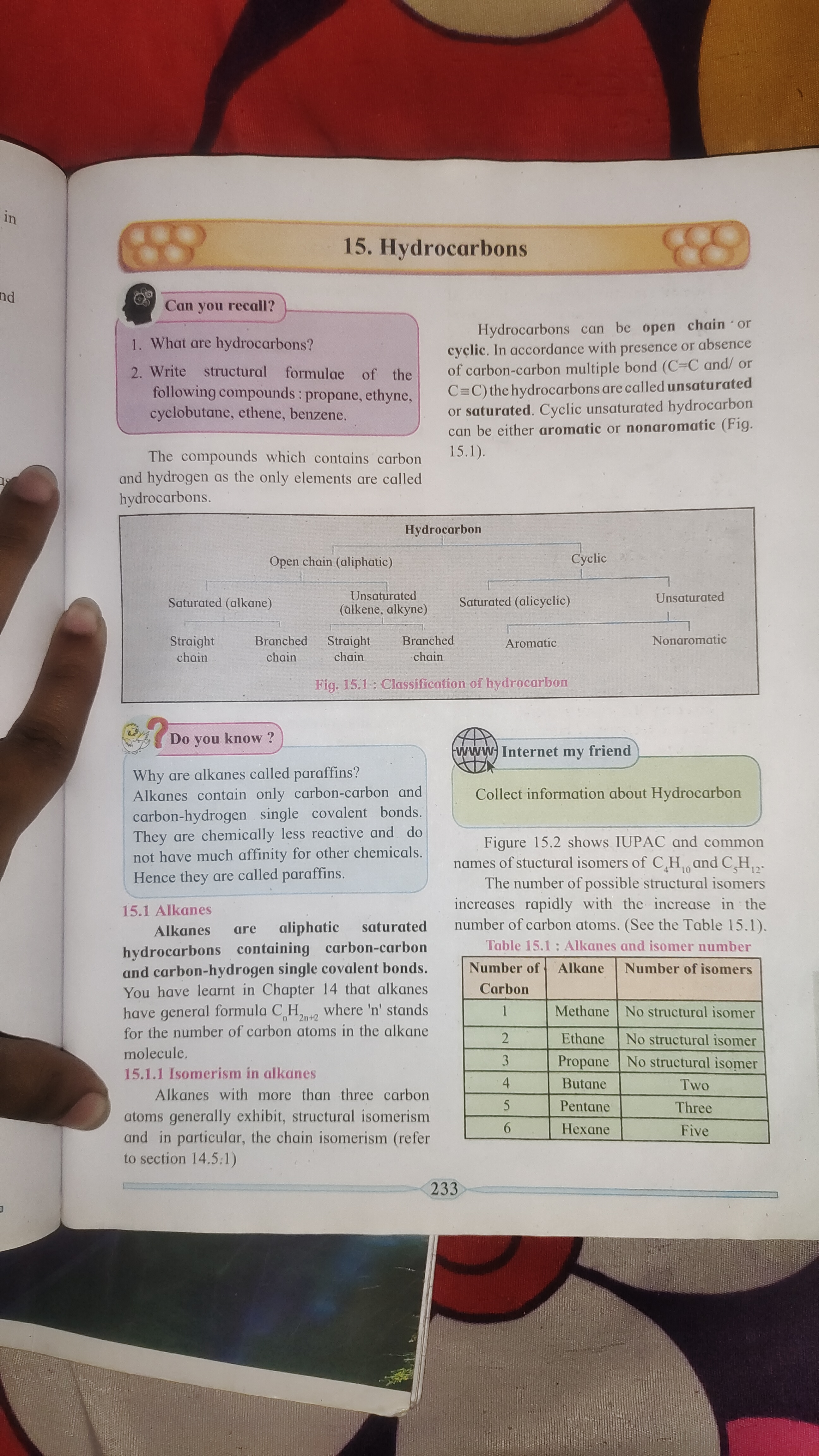
Hydrocarbons are organic compounds consisting entirely of hydrogen and carbon. They can be classified as aliphatic or cyclic, and further divided into saturated (alkanes) or unsaturated (alkenes and alkynes).
Tips
Ensure correct bond placement when drawing structural formulas. Mistakes often occur in placing double or triple bonds.
Sources
- Write structural formulae of the following compounds - sarthaks.com
- Write structural formulae - EMBIBE - embibe.com
AI-generated content may contain errors. Please verify critical information