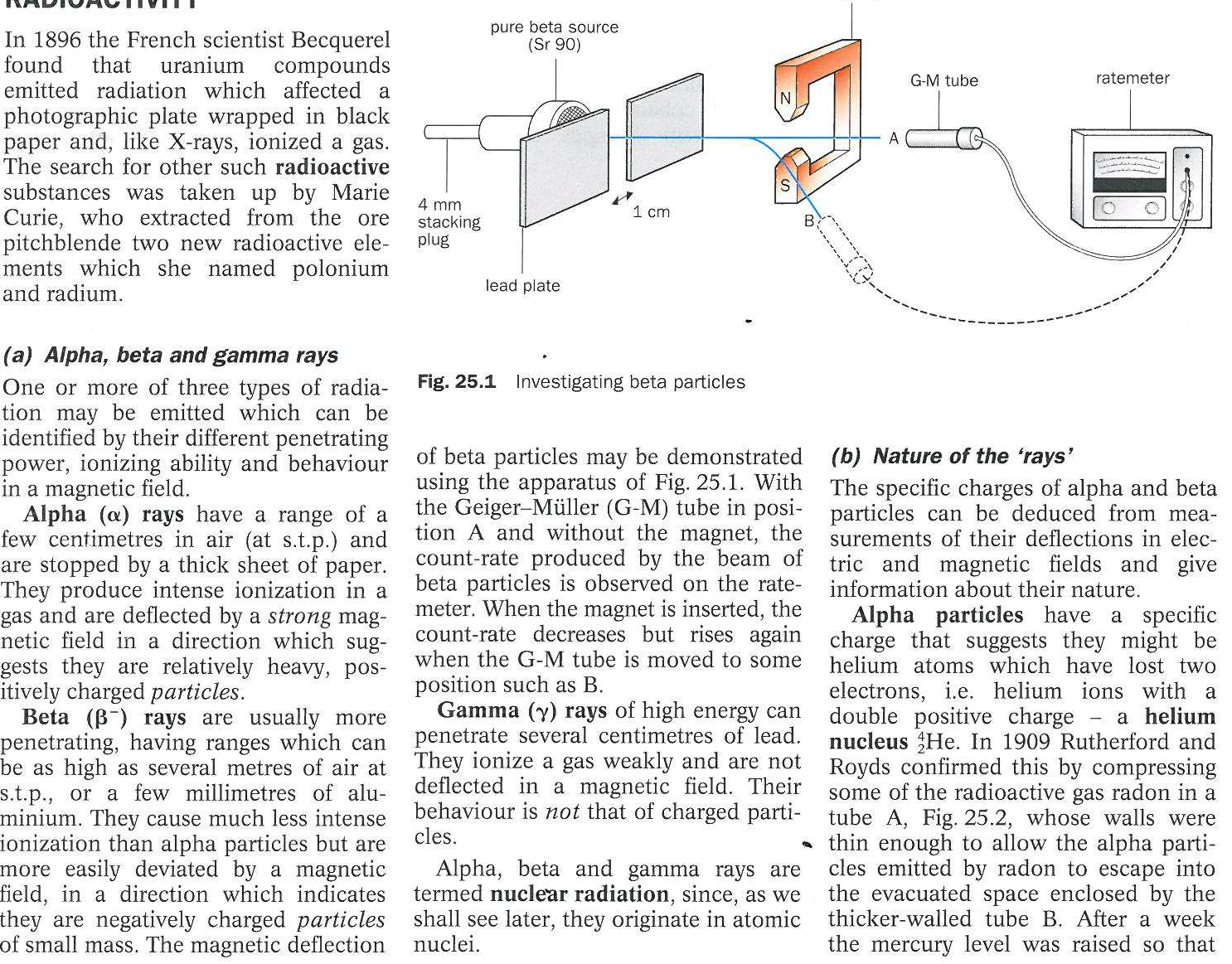
What are alpha, beta, and gamma rays, and how can they be identified?
Understand the Problem
The question is discussing the types of radiation and their characteristics, specifically alpha, beta, and gamma rays, as well as their behavior in magnetic fields and how they are detected.
Answer
Alpha are helium nuclei, beta are electrons, gamma are photons; identified by penetration and deflection.
Alpha rays are helium nuclei, beta rays are high-speed electrons, and gamma rays are high-energy photons. They are identified through their penetrating abilities and magnetic properties.
Answer for screen readers
Alpha rays are helium nuclei, beta rays are high-speed electrons, and gamma rays are high-energy photons. They are identified through their penetrating abilities and magnetic properties.
More Information
Alpha particles are positively charged and stopped by paper, beta particles are negatively charged and more penetrating, while gamma rays are neutral energy photons requiring thick lead for stopping.
Tips
Confusing the mass and energy of particles can lead to errors. Remember alpha particles are the heaviest, gamma rays have no mass.
Sources
AI-generated content may contain errors. Please verify critical information